Pheromone binding protein: Difference between revisions
Nurit Eliash (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (15 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
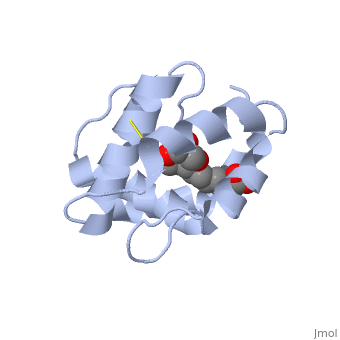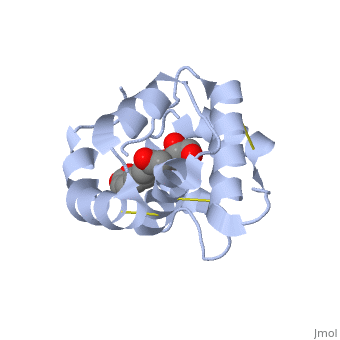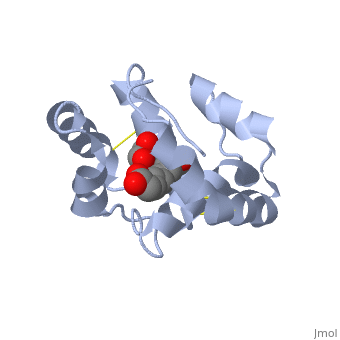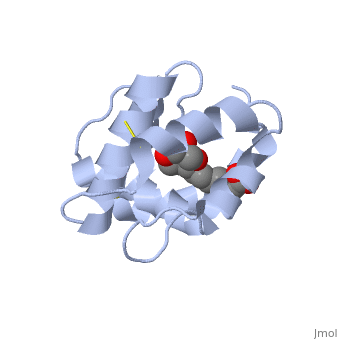
<StructureSection load='3bfa' size='340' side='right' caption='Pheromone binding protein of honey bee complex with pheromone (PDB code [[3bfa]]).' scene=''> | |||
__TOC__ | |||
==Introduction== | ==Introduction== | ||
'''Pheromone binding proteins''' [http://en.wikipedia.org/wiki/Pheromone_binding_protein (PBP)] are type of Odorant binding proteins [http://en.wikipedia.org/wiki/Odorant-binding_protein (OBP)] - soluble proteins mediating the early stages of volatiles detection in both insects and vertebrates<ref>DOI:10.3389/fphys.2014.00320</ref>. The volatiles (pheromones and other small hydrophobic molecules) are solubilized by the OBPs and activate the chemoreceptors. | |||
The | |||
As a model protein of this family we will further describe the well investigated Pheromone binding protein of the honey bee, ASP1. | |||
As a model protein of this family we will further describe the well investigated Pheromone binding protein of the honey bee. | |||
== Pheromone-binding protein ASP1 == | |||
Chemical communication is crucial in social insects, where a complicated and delicate system of signals must be maintained in order to preserve the fragile equilibrium between the queen and the workers. In the hive of the honey bee [http://en.wikipedia.org/wiki/Apis_mellifera ''Apis mellifera''] this equilibrium exists partially due to the extraction of blend of substances called queen mandibular pheromone [http://en.wikipedia.org/wiki/Honey_bee_pheromones#Queen_mandibular_pheromone (QMP)], by the queen <ref>Winston, M.L., 1987. The Biology of the Honey Bee. Harvard University Press, Cambridge, MA.</ref>. The three major component of the QMP blend are: 9-keto-2(E)-decenoic acid (9-ODA) and 9-hydroxy-2(E)-decenoic acid (9-HDA R-(−) or S-(+)). | |||
Pheromone-binding protein [http://www.uniprot.org/uniprot/Q9U9J6/ ASP1] of the honeybee [http://en.wikipedia.org/wiki/Apis_mellifera ''Apis mellifera''] L. (Hymenoptera: Apidea) was first isolated and characterized by Danty ''et al''. (1998)<ref>DOI:10.1016/j.jmb.2008.04.048</ref> from the bee antennae. | |||
== Structure == | |||
<scene name='60/609542/Disulfide_bonds/1'>3 disulfide bonds</scene> | The protein is composed of 144 amino acids, which forms 6 alpha helices. Three <scene name='60/609542/Disulfide_bonds/1'>3 disulfide bonds</scene> formed by 6 Cystein residues tied four helices: disulfide 20–51 between H1 and H3, 47– 98 between H3 and H6, and 107–89 between H6 and H5. | ||
<scene name='60/609542/Binding_site/ | == Interaction with the ligand 9-ODA== | ||
One of the main components of the QMP <scene name='60/609542/9-oda/3'>9-ODA</scene>, is binding to the protein binding site along with a <scene name='60/609542/Glycerol/2'>glycerol molecule</scene>.The carboxyl end of 9-ODA points towards the solvent, and has no bonds with residues of the protein. The residues in the binding site are <scene name='60/609542/Binding_site/3'>hydrophobic</scene>, and the connection between 9-ODA and ASP1 involve hydrogen bonds. | |||
</StructureSection> | |||
== | ==3D structures of pheromone-binding protein== | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | |||
{{#tree:id=OrganizedByTopic|openlevels=0| | |||
* Pheromone binding protein | |||
**[[2h8v]], [[3bjh]], [[3cab]], [[3cdn]], [[3cz2]] – bPBP residues 26-144 – honey bee<br /> | |||
**[[3d73]], [[3d74]], [[3d75]], [[3d76]], [[3d77]], [[3d78]] - bPBP residues 26-144 (mutant)<br /> | |||
**[[1dqe]], [[2fjy]] – sPBP – silkworm<br /> | |||
**[[1xfr]] – sPBP – NMR<br /> | |||
**[[1gm0]] – sPBP (mutant) – NMR<br /> | |||
**[[1qwv]], [[1two]], [[2jpo]], [[6um9]] – mPBP – moth - NMR<br /> | |||
**[[6vq5]] – mPBP <br /> | |||
**[[7uo6]] – PBP2 – corn borer - NMR<br /> | |||
**[[7vw8]], [[7vw9]] – bwPBP1 - bollworm<br /> | |||
* Pheromone binding protein complex | |||
**[[3bfa]], [[3bfb]], [[3bfh]], [[3cyz]] – bPBP residues 26-144 + pheromone<br /> | |||
**[[3cz0]], [[3cz1]] - bPBP residues 26-144 + N-butyl benzene sulfonamide<br /> | |||
**[[3fe6]], [[3fe8]], [[3fe9]] - bPBP residues 26-144 + methyldotetracontane<br /> | |||
**[[2p70]] – sPBP + odorant<br /> | |||
**[[2p71]] – sPBP + iodohexadecane<br /> | |||
**[[4inw]], [[4inx]] – PBP + hexadecadienal – ''Amyelois transitella''<br /> | |||
**[[7vwa]] – bwPBP1 + odorant <br /> | |||
}} | |||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category: Topic Page]] | |||
Latest revision as of 12:40, 23 August 2023
IntroductionPheromone binding proteins (PBP) are type of Odorant binding proteins (OBP) - soluble proteins mediating the early stages of volatiles detection in both insects and vertebrates[1]. The volatiles (pheromones and other small hydrophobic molecules) are solubilized by the OBPs and activate the chemoreceptors. As a model protein of this family we will further describe the well investigated Pheromone binding protein of the honey bee, ASP1. Pheromone-binding protein ASP1Chemical communication is crucial in social insects, where a complicated and delicate system of signals must be maintained in order to preserve the fragile equilibrium between the queen and the workers. In the hive of the honey bee Apis mellifera this equilibrium exists partially due to the extraction of blend of substances called queen mandibular pheromone (QMP), by the queen [2]. The three major component of the QMP blend are: 9-keto-2(E)-decenoic acid (9-ODA) and 9-hydroxy-2(E)-decenoic acid (9-HDA R-(−) or S-(+)). Pheromone-binding protein ASP1 of the honeybee Apis mellifera L. (Hymenoptera: Apidea) was first isolated and characterized by Danty et al. (1998)[3] from the bee antennae. StructureThe protein is composed of 144 amino acids, which forms 6 alpha helices. Three formed by 6 Cystein residues tied four helices: disulfide 20–51 between H1 and H3, 47– 98 between H3 and H6, and 107–89 between H6 and H5. Interaction with the ligand 9-ODAOne of the main components of the QMP , is binding to the protein binding site along with a .The carboxyl end of 9-ODA points towards the solvent, and has no bonds with residues of the protein. The residues in the binding site are , and the connection between 9-ODA and ASP1 involve hydrogen bonds.
|
| ||||||||||
3D structures of pheromone-binding protein3D structures of pheromone-binding protein
Updated on 23-August-2023
ReferencesReferences
- ↑ Pelosi P, Iovinella I, Felicioli A, Dani FR. Soluble proteins of chemical communication: an overview across arthropods. Front Physiol. 2014 Aug 27;5:320. doi: 10.3389/fphys.2014.00320. eCollection, 2014. PMID:25221516 doi:http://dx.doi.org/10.3389/fphys.2014.00320
- ↑ Winston, M.L., 1987. The Biology of the Honey Bee. Harvard University Press, Cambridge, MA.
- ↑ Pesenti ME, Spinelli S, Bezirard V, Briand L, Pernollet JC, Tegoni M, Cambillau C. Structural basis of the honey bee PBP pheromone and pH-induced conformational change. J Mol Biol. 2008 Jun 27;380(1):158-69. Epub 2008 Apr 27. PMID:18508083 doi:10.1016/j.jmb.2008.04.048