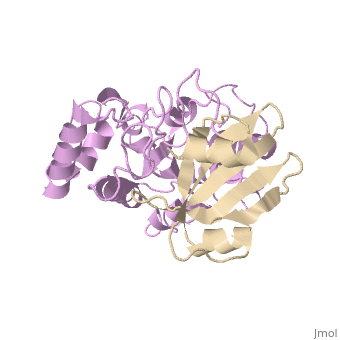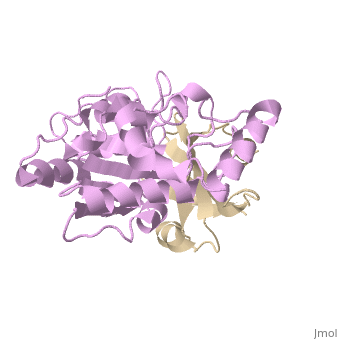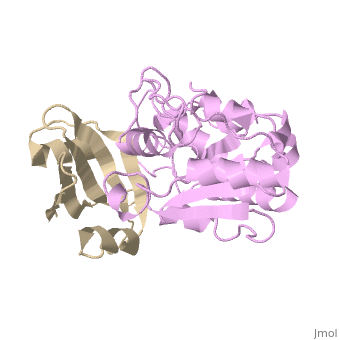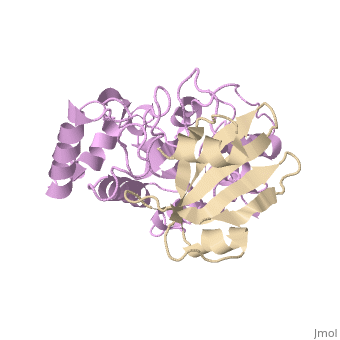1ugh: Difference between revisions
No edit summary |
No edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== | ==CRYSTAL STRUCTURE OF HUMAN URACIL-DNA GLYCOSYLASE IN COMPLEX WITH A PROTEIN INHIBITOR: PROTEIN MIMICRY OF DNA== | ||
[[1ugh]] is a 2 chain structure with sequence from [ | <StructureSection load='1ugh' size='340' side='right'caption='[[1ugh]], [[Resolution|resolution]] 1.90Å' scene=''> | ||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1ugh]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Bacillus_phage_PBS2 Bacillus phage PBS2] and [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1UGH OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1UGH FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.9Å</td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1ugh FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1ugh OCA], [https://pdbe.org/1ugh PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1ugh RCSB], [https://www.ebi.ac.uk/pdbsum/1ugh PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1ugh ProSAT]</span></td></tr> | |||
</table> | |||
== Disease == | |||
[https://www.uniprot.org/uniprot/UNG_HUMAN UNG_HUMAN] Defects in UNG are a cause of immunodeficiency with hyper-IgM type 5 (HIGM5) [MIM:[https://omim.org/entry/608106 608106]. A rare immunodeficiency syndrome characterized by normal or elevated serum IgM levels with absence of IgG, IgA, and IgE. It results in a profound susceptibility to bacterial infections.<ref>PMID:12958596</ref> <ref>PMID:15967827</ref> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/UNG_HUMAN UNG_HUMAN] Excises uracil residues from the DNA which can arise as a result of misincorporation of dUMP residues by DNA polymerase or due to deamination of cytosine. | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/ug/1ugh_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1ugh ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Uracil-DNA glycosylase inhibitor (Ugi) is a B. subtilis bacteriophage protein that protects the uracil-containing phage DNA by irreversibly inhibiting the key DNA repair enzyme uracil-DNA glycosylase (UDG). The 1.9 A crystal structure of Ugi complexed to human UDG reveals that the Ugi structure, consisting of a twisted five-stranded antiparallel beta sheet and two alpha helices, binds by inserting a beta strand into the conserved DNA-binding groove of the enzyme without contacting the uracil specificity pocket. The resulting interface, which buries over 1200 A2 on Ugi and involves the entire beta sheet and an alpha helix, is polar and contains 22 water molecules. Ugi binds the sequence-conserved DNA-binding groove of UDG via shape and electrostatic complementarity, specific charged hydrogen bonds, and hydrophobic packing enveloping Leu-272 from a protruding UDG loop. The apparent mimicry by Ugi of DNA interactions with UDG provides both a structural mechanism for UDG binding to DNA, including the enzyme-assisted expulsion of uracil from the DNA helix, and a crystallographic basis for the design of inhibitors with scientific and therapeutic applications. | |||
Crystal structure of human uracil-DNA glycosylase in complex with a protein inhibitor: protein mimicry of DNA.,Mol CD, Arvai AS, Sanderson RJ, Slupphaug G, Kavli B, Krokan HE, Mosbaugh DW, Tainer JA Cell. 1995 Sep 8;82(5):701-8. PMID:7671300<ref>PMID:7671300</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1ugh" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
*[[DNA | *[[DNA glycosylase 3D structures|DNA glycosylase 3D structures]] | ||
*[[Uracil | *[[Uracil glycosylase inhibitor|Uracil glycosylase inhibitor]] | ||
== References == | |||
== | <references/> | ||
< | __TOC__ | ||
[[Category: Bacillus phage | </StructureSection> | ||
[[Category: Bacillus phage PBS2]] | |||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: Arvai | [[Category: Arvai AS]] | ||
[[Category: Kavli | [[Category: Kavli B]] | ||
[[Category: Krokan | [[Category: Krokan HE]] | ||
[[Category: Mol | [[Category: Mol CD]] | ||
[[Category: Mosbaugh | [[Category: Mosbaugh DW]] | ||
[[Category: Sanderson | [[Category: Sanderson RJ]] | ||
[[Category: Slupphaug | [[Category: Slupphaug G]] | ||
[[Category: Tainer | [[Category: Tainer JA]] | ||
Latest revision as of 09:40, 23 August 2023
CRYSTAL STRUCTURE OF HUMAN URACIL-DNA GLYCOSYLASE IN COMPLEX WITH A PROTEIN INHIBITOR: PROTEIN MIMICRY OF DNACRYSTAL STRUCTURE OF HUMAN URACIL-DNA GLYCOSYLASE IN COMPLEX WITH A PROTEIN INHIBITOR: PROTEIN MIMICRY OF DNA
Structural highlights
DiseaseUNG_HUMAN Defects in UNG are a cause of immunodeficiency with hyper-IgM type 5 (HIGM5) [MIM:608106. A rare immunodeficiency syndrome characterized by normal or elevated serum IgM levels with absence of IgG, IgA, and IgE. It results in a profound susceptibility to bacterial infections.[1] [2] FunctionUNG_HUMAN Excises uracil residues from the DNA which can arise as a result of misincorporation of dUMP residues by DNA polymerase or due to deamination of cytosine. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedUracil-DNA glycosylase inhibitor (Ugi) is a B. subtilis bacteriophage protein that protects the uracil-containing phage DNA by irreversibly inhibiting the key DNA repair enzyme uracil-DNA glycosylase (UDG). The 1.9 A crystal structure of Ugi complexed to human UDG reveals that the Ugi structure, consisting of a twisted five-stranded antiparallel beta sheet and two alpha helices, binds by inserting a beta strand into the conserved DNA-binding groove of the enzyme without contacting the uracil specificity pocket. The resulting interface, which buries over 1200 A2 on Ugi and involves the entire beta sheet and an alpha helix, is polar and contains 22 water molecules. Ugi binds the sequence-conserved DNA-binding groove of UDG via shape and electrostatic complementarity, specific charged hydrogen bonds, and hydrophobic packing enveloping Leu-272 from a protruding UDG loop. The apparent mimicry by Ugi of DNA interactions with UDG provides both a structural mechanism for UDG binding to DNA, including the enzyme-assisted expulsion of uracil from the DNA helix, and a crystallographic basis for the design of inhibitors with scientific and therapeutic applications. Crystal structure of human uracil-DNA glycosylase in complex with a protein inhibitor: protein mimicry of DNA.,Mol CD, Arvai AS, Sanderson RJ, Slupphaug G, Kavli B, Krokan HE, Mosbaugh DW, Tainer JA Cell. 1995 Sep 8;82(5):701-8. PMID:7671300[3] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||