1a72: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==AN ACTIVE-SITE DOUBLE MUTANT (PHE93->TRP, VAL203->ALA) OF HORSE LIVER ALCOHOL DEHYDROGENASE IN COMPLEX WITH THE ISOSTERIC NAD ANALOG CPAD== | |||
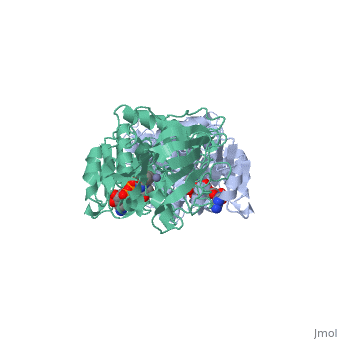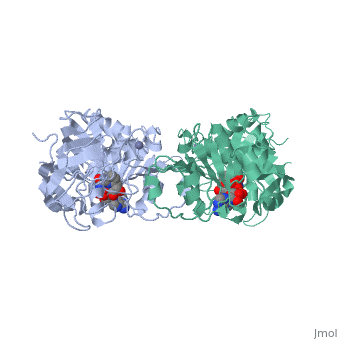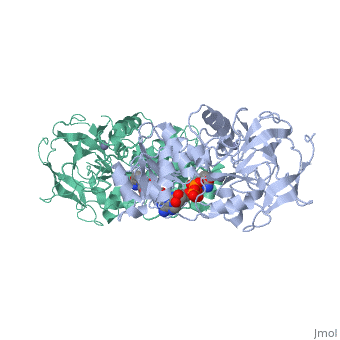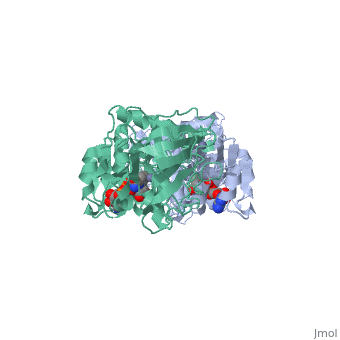
<StructureSection load='1a72' size='340' side='right'caption='[[1a72]], [[Resolution|resolution]] 2.60Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1a72]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Equus_caballus Equus caballus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1A72 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1A72 FirstGlance]. <br> | |||
</td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.6Å</td></tr> | |||
<tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=PAD:5-BETA-D-RIBOFURANOSYLPICOLINAMIDE+ADENINE-DINUCLEOTIDE'>PAD</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | |||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1a72 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1a72 OCA], [https://pdbe.org/1a72 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1a72 RCSB], [https://www.ebi.ac.uk/pdbsum/1a72 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1a72 ProSAT]</span></td></tr> | |||
</table> | |||
== Function == | |||
[https://www.uniprot.org/uniprot/ADH1E_HORSE ADH1E_HORSE] | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/a7/1a72_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=1a72 ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
The oxidation of alcohol to aldehyde by horse liver alcohol dehydrogenase (LADH) requires the transfer of a hydride ion from the alcohol substrate to the cofactor nicotinamide adenine dinucleotide (NAD). A quantum mechanical tunneling contribution to this hydride transfer step has been demonstrated in a number of LADH mutants designed to enhance or diminish this effect [Bahnson, B. J., et al. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 12797-12802]. The active site double mutant Phe93 --> Trp/Val203 --> Ala shows a 75-fold reduction in catalytic efficiency relative to that of the native enzyme, and reduced tunneling relative to that of either single mutant. We present here two crystal structures of the double mutant: a 2.0 A complex with NAD and the substrate analogue trifluoroethanol and a 2.6 A complex with the isosteric NAD analogue CPAD and ethanol. Changes at the active site observed in both complexes are consistent with reduced activity and tunneling. The NAD-trifluoroethanol complex crystallizes in the closed conformation characteristic of the active enzyme. However, the NAD nicotinamide ring rotates away from the substrate, toward the space vacated by replacement of Val203 with the smaller alanine. Replacement of Phe93 with the larger tryptophan also produces unfavorable steric contacts with the nicotinamide carboxamide group, potentially destabilizing hydrogen bonds required to maintain the closed conformation. These contacts are relieved in the second complex by rotation of the CPAD pyridine ring into an unusual syn orientation. The resulting loss of the carboxamide hydrogen bonds produces an open conformation characteristic of the apoenzyme. | |||
Active site modifications in a double mutant of liver alcohol dehydrogenase: structural studies of two enzyme-ligand complexes.,Colby TD, Bahnson BJ, Chin JK, Klinman JP, Goldstein BM Biochemistry. 1998 Jun 30;37(26):9295-304. PMID:9649310<ref>PMID:9649310</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
<div class="pdbe-citations 1a72" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
*[[Alcohol dehydrogenase|Alcohol dehydrogenase]] | *[[Alcohol dehydrogenase 3D structures|Alcohol dehydrogenase 3D structures]] | ||
== References == | |||
<references/> | |||
== | __TOC__ | ||
< | </StructureSection> | ||
[[Category: Equus caballus]] | [[Category: Equus caballus]] | ||
[[Category: | [[Category: Large Structures]] | ||
[[Category: | [[Category: Bahnson BJ]] | ||
[[Category: | [[Category: Chin JK]] | ||
[[Category: | [[Category: Colby TD]] | ||
[[Category: | [[Category: Goldstein BM]] | ||
[[Category: | [[Category: Klinman JP]] | ||
Latest revision as of 13:48, 2 August 2023
AN ACTIVE-SITE DOUBLE MUTANT (PHE93->TRP, VAL203->ALA) OF HORSE LIVER ALCOHOL DEHYDROGENASE IN COMPLEX WITH THE ISOSTERIC NAD ANALOG CPADAN ACTIVE-SITE DOUBLE MUTANT (PHE93->TRP, VAL203->ALA) OF HORSE LIVER ALCOHOL DEHYDROGENASE IN COMPLEX WITH THE ISOSTERIC NAD ANALOG CPAD
Structural highlights
FunctionEvolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe oxidation of alcohol to aldehyde by horse liver alcohol dehydrogenase (LADH) requires the transfer of a hydride ion from the alcohol substrate to the cofactor nicotinamide adenine dinucleotide (NAD). A quantum mechanical tunneling contribution to this hydride transfer step has been demonstrated in a number of LADH mutants designed to enhance or diminish this effect [Bahnson, B. J., et al. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 12797-12802]. The active site double mutant Phe93 --> Trp/Val203 --> Ala shows a 75-fold reduction in catalytic efficiency relative to that of the native enzyme, and reduced tunneling relative to that of either single mutant. We present here two crystal structures of the double mutant: a 2.0 A complex with NAD and the substrate analogue trifluoroethanol and a 2.6 A complex with the isosteric NAD analogue CPAD and ethanol. Changes at the active site observed in both complexes are consistent with reduced activity and tunneling. The NAD-trifluoroethanol complex crystallizes in the closed conformation characteristic of the active enzyme. However, the NAD nicotinamide ring rotates away from the substrate, toward the space vacated by replacement of Val203 with the smaller alanine. Replacement of Phe93 with the larger tryptophan also produces unfavorable steric contacts with the nicotinamide carboxamide group, potentially destabilizing hydrogen bonds required to maintain the closed conformation. These contacts are relieved in the second complex by rotation of the CPAD pyridine ring into an unusual syn orientation. The resulting loss of the carboxamide hydrogen bonds produces an open conformation characteristic of the apoenzyme. Active site modifications in a double mutant of liver alcohol dehydrogenase: structural studies of two enzyme-ligand complexes.,Colby TD, Bahnson BJ, Chin JK, Klinman JP, Goldstein BM Biochemistry. 1998 Jun 30;37(26):9295-304. PMID:9649310[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||