Cytochrome bc1 complex: Difference between revisions
No edit summary |
No edit summary |
||
| (18 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
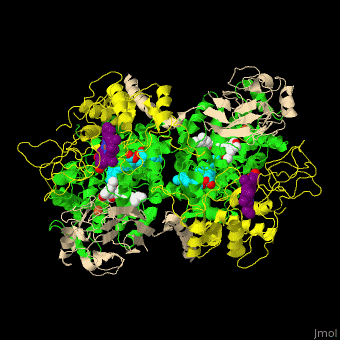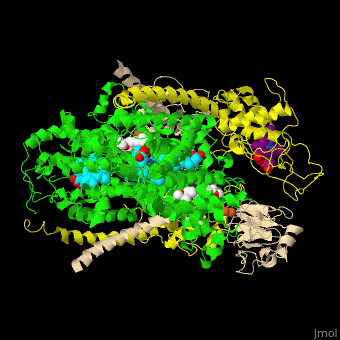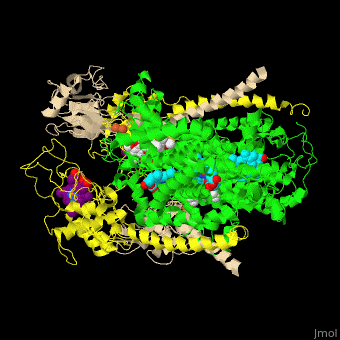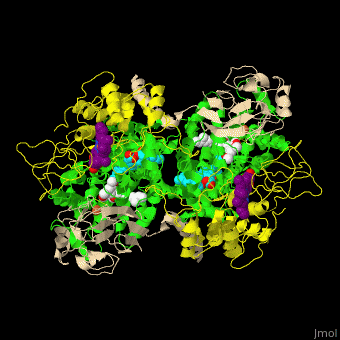
<StructureSection load=' | <StructureSection load='' size='350' side='right' caption='Cytochrome bc1 containing Cb (green), Cc1 (yellow), RISP (beige) and Fe2S2 complex with stigmatellin (PDB entry [[1zrt]])' scene='49/490879/Cv/6'> | ||
'''Cytochrome bc1''' (Cbc1) functions as the central pump which transfers protons across the cell membrane. The protons are used to power the rotation of ATP synthase. Cbc1 binds ubiquinol which carries hydrogen atoms. Cbc1 separates the protons and the electrons. The protons are released in the inner side of the membrane for use by ATP synthase and the electrons are transferred to cytochrome c or to the outer side of the membrane. Plants use '''cytochrome b6f''' in the same manner binding plastoquinol as a hydrogen carrier. Stigmatellin inhibits the Cbc1 electron transfer by binding to its quinone oxidation site. Antimycin inhibits Cbc1 by binding to its quinone reduction site.<ref>PMID:14977419</ref> | '''Cytochrome bc1''' (Cbc1) functions as the central pump which transfers protons across the cell membrane. The protons are used to power the rotation of ATP synthase. Cbc1 binds ubiquinol which carries hydrogen atoms. Cbc1 separates the protons and the electrons. The protons are released in the inner side of the membrane for use by ATP synthase and the electrons are transferred to cytochrome c or to the outer side of the membrane. Plants use '''cytochrome b6f''' in the same manner binding plastoquinol as a hydrogen carrier. Stigmatellin inhibits the Cbc1 electron transfer by binding to its quinone oxidation site. Antimycin inhibits Cbc1 by binding to its quinone reduction site.<ref>PMID:14977419</ref> | ||
More details in [[ | More details in [[Complex III of Electron Transport Chain]]. | ||
== Structural highlights == | == Structural highlights == | ||
Cbc1 is a dimeric protein composed of 11 proteins and cofactors which include heme-carrying proteins like '' | Cbc1 is a <scene name='49/490879/Cv/11'>dimeric protein</scene> composed of 11 proteins and cofactors which include heme-carrying proteins like <scene name='49/490879/Cv/12'>cytochrome b (Cb)</scene> and <scene name='49/490879/Cv/13'>cytochrome c1 (Cc1)</scene> and iron-sulfur cluster proteins like <scene name='49/490879/Cv/14'>Rieske Fe-S protein (RISP)</scene>. The iron containing moieties are <scene name='49/490879/Cv/15'>heme</scene>, <scene name='49/490879/Cv/16'>heme C</scene> (where vinyl side chain of heme are replaced by thioether) and <scene name='49/490879/Cv/17'>Fe2S2</scene>. <ref>PMID:16034531</ref> | ||
</StructureSection> | </StructureSection> | ||
==3D structures of cytochrome bc1== | ==3D structures of cytochrome bc1== | ||
[[Cytochrome bc1 3D structures]] | |||
[[ | |||
== References == | == References == | ||
Latest revision as of 13:45, 12 February 2023
Cytochrome bc1 (Cbc1) functions as the central pump which transfers protons across the cell membrane. The protons are used to power the rotation of ATP synthase. Cbc1 binds ubiquinol which carries hydrogen atoms. Cbc1 separates the protons and the electrons. The protons are released in the inner side of the membrane for use by ATP synthase and the electrons are transferred to cytochrome c or to the outer side of the membrane. Plants use cytochrome b6f in the same manner binding plastoquinol as a hydrogen carrier. Stigmatellin inhibits the Cbc1 electron transfer by binding to its quinone oxidation site. Antimycin inhibits Cbc1 by binding to its quinone reduction site.[1] More details in Complex III of Electron Transport Chain. Structural highlightsCbc1 is a composed of 11 proteins and cofactors which include heme-carrying proteins like and and iron-sulfur cluster proteins like . The iron containing moieties are , (where vinyl side chain of heme are replaced by thioether) and . [2] |
| ||||||||||
3D structures of cytochrome bc13D structures of cytochrome bc1
ReferencesReferences
- ↑ Crofts AR. The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol. 2004;66:689-733. PMID:14977419 doi:http://dx.doi.org/10.1146/annurev.physiol.66.032102.150251
- ↑ Berry EA, Huang LS, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F. X-Ray Structure of Rhodobacter Capsulatus Cytochrome bc (1): Comparison with its Mitochondrial and Chloroplast Counterparts. Photosynth Res. 2004;81(3):251-75. PMID:16034531 doi:http://dx.doi.org/10.1023/B:PRES.0000036888.18223.0e