Sulfhydryl oxidase: Difference between revisions
Jump to navigation
Jump to search
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
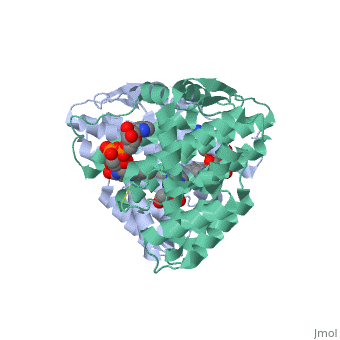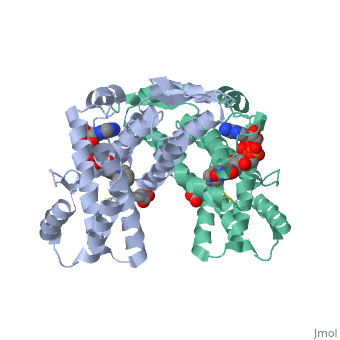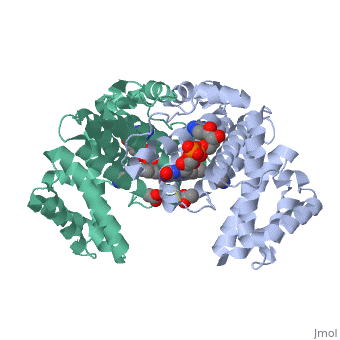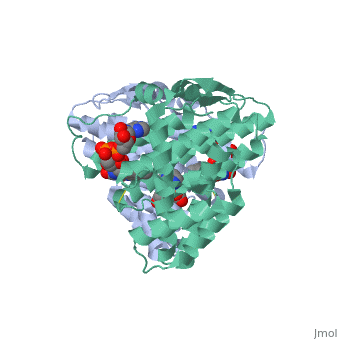
<StructureSection load='3p0k' size='350' side='right' scene='' caption='Baculovirus sulfhydryl oxidase complex with acetate and imidazole, showing the FAD, [[3p0k]]'> | |||
'''Sulfhydryl oxidase''' (SOX) is a flavin-dependent enzyme which catalyzes the formation of disulfide bonds from thiol groups. The reaction involves the reduction of O<sub>2</sub> to hydrogen peroxide<ref>PMID:12176051</ref>. | |||
*'''Erv1p''' is involved in the biogenesis of Fe/S clusters<ref>PMID:10899311</ref>.<br /> | |||
*'''ALR''' is a SOX augmenter of liver regeneration<ref>PMID:23186364</ref>.<br /> | |||
*'''QSOX''' is a quiescin SOX. QSOX contains thioredoxin domain, helix-rich domain and FAD-binding domain Erv/ALR<ref>PMID:18393449</ref>. | |||
*<scene name='46/468158/Cv/3'>FAD binding site</scene> in Baculovirus sulfhydryl oxidase ([[3p0k]]). Water molecules are shown as red spheres. | |||
==3D structures of sulfhydryl oxidase== | |||
[[Sulfhydryl oxidase 3D structures]] | |||
</StructureSection> | |||
== References == | |||
<references/> | |||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Latest revision as of 12:56, 11 February 2020
Sulfhydryl oxidase (SOX) is a flavin-dependent enzyme which catalyzes the formation of disulfide bonds from thiol groups. The reaction involves the reduction of O2 to hydrogen peroxide[1].
3D structures of sulfhydryl oxidaseSulfhydryl oxidase 3D structures
|
| ||||||||||
ReferencesReferences
- ↑ Thorpe C, Hoober KL, Raje S, Glynn NM, Burnside J, Turi GK, Coppock DL. Sulfhydryl oxidases: emerging catalysts of protein disulfide bond formation in eukaryotes. Arch Biochem Biophys. 2002 Sep 1;405(1):1-12. PMID:12176051
- ↑ Lee J, Hofhaus G, Lisowsky T. Erv1p from Saccharomyces cerevisiae is a FAD-linked sulfhydryl oxidase. FEBS Lett. 2000 Jul 14;477(1-2):62-6. PMID:10899311
- ↑ Sztolsztener ME, Brewinska A, Guiard B, Chacinska A. Disulfide bond formation: sulfhydryl oxidase ALR controls mitochondrial biogenesis of human MIA40. Traffic. 2013 Mar;14(3):309-20. doi: 10.1111/tra.12030. Epub 2012 Dec 16. PMID:23186364 doi:http://dx.doi.org/10.1111/tra.12030
- ↑ Heckler EJ, Alon A, Fass D, Thorpe C. Human quiescin-sulfhydryl oxidase, QSOX1: probing internal redox steps by mutagenesis. Biochemistry. 2008 Apr 29;47(17):4955-63. doi: 10.1021/bi702522q. Epub 2008 Apr, 5. PMID:18393449 doi:http://dx.doi.org/10.1021/bi702522q