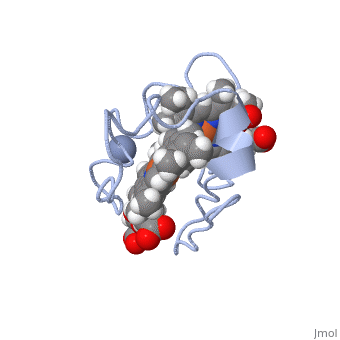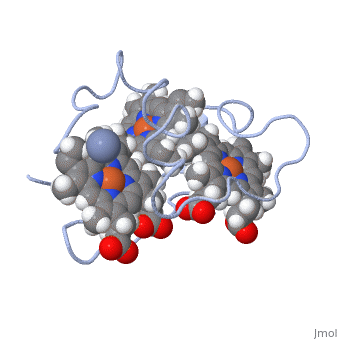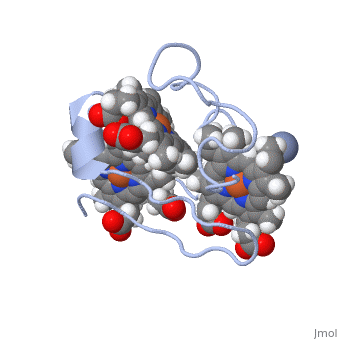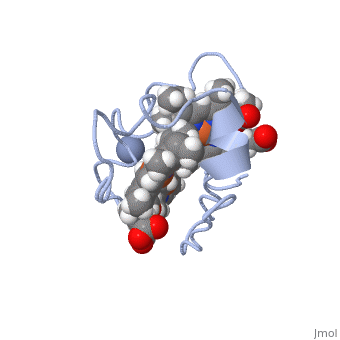Cytochrome c 7: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
==General== | ==General== | ||
<StructureSection load='1LM2.pdb' size='340' side='right' caption='3D Structure of Cytochrome c | <StructureSection load='1LM2.pdb' size='340' side='right' caption='3D Structure of Cytochrome c 7with heme and Cr+3 ion (PDB code [[1lm2]])' scene=''> | ||
'''Cytocrhome c 7''' (Cc7) is a three heme-containing protein derived from the sulfur-reducing bacterium ''Desulfuromonas acetoxidans''. Cc7 is crucial to the bacteria's anaerobic | '''Cytocrhome c 7''' (Cc7) is a three heme-containing protein derived from the sulfur-reducing bacterium ''Desulfuromonas acetoxidans''. Cc7 is crucial to the bacteria's anaerobic sulfur respiration as it plays a role in the electron-transfer mechanism, and as such it is located in the mitochondrial inter-membrane space<ref name="assfalt">Assfalg M, Bertini I, Bruschi M, Michel C, Turano P. The metal reductase activity of some multiheme cytochromes c: NMR structural characterization of the reduction of chromium(VI) to chromium(III) by cytochrome c(7). 2002; 99(15):9750-4 '''[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC125002/''' DOI: 10.1073/pnas.152290999''']'''</ref>. Over the course of 3.5 million years, ''Desulfuromonas acetoxidans'' have evolved to use anaerobic sulfur respiration as the main driving force of their survival<ref>Barton L, Fauque G. Biochemistry, Physiology and Biotechnology of Sulfate-Reducing Bacteria. Advances in Applied Microbiology. 2009; 68: 41–98. '''[http://www.sciencedirect.com/science/article/pii/S0065216409012027''' DOI: 10.1016/s0065-2164(09)01202-7''']'''</ref>. | ||
As a result, Cc7 is able to reduce sulfur, its oxidized variants (thiosulfate, sulfite, sulfate, etc) and even heavy metals to produce the required energy<ref name="pennies">Pfennig N, Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. 1976; 110(1): 3-12 '''[http://link.springer.com/article/10.1007/BF00416962''' DOI: 10.1007/BF00416962''']'''</ref>. If using sulfur, the product formed is hydrogen sulfide. This compound is able to react with heavy metal ions to form almost non-lethal and quite insoluble metal sulfides<ref name="assfalt" />. This enzymatic ability of Cc7 is unique to the animal kingdom, and the ability to create less-toxic and insoluble metal sulfides has intrigued researchers as of late<ref name="pennies">. | |||
By harnessing ''Desulfuromonas acetoxidans'' and its Cc7 protein, researchers hope to create possible applications to use these bacteria to decontaminate environments polluted with toxic heavy metals from industrial wastes across the globe. And because of the metal sulfides insolubility, removing them from the environment will be simpler and cheaper than current heavy metal toxic waste filtration operations<ref>National Service Center for Environmental Publications. [http://nepis.epa.gov/Exe/ZyNET.exe/91018HWZ.txt?ZyActionD=ZyDocument&Client=EPA&Index=1976%20Thru%201980&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A\ZYFILES\INDEX%20DATA\76THRU80\TXT\00000025\91018HWZ.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h|-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=p|f&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1]</ref>. | |||
==Structural Components== | ==Structural Components== | ||
Cc7 is a single polypeptide chain 68 residues total containing three heme groups. The polypeptide strand has one alpha helix 4 residues in length and two beta strands 2 residues in length. The heme groups are labelled as I, III, and IV. The binding site is located on the distal side of heme group IV where <scene name='71/716635/ | Cc7 is a single polypeptide chain 68 residues total containing three heme groups. The polypeptide strand has one alpha helix 4 residues in length and two beta strands 2 residues in length. The heme groups are labelled <scene name='71/716635/Heme_groups/2'>as I, III, and IV</scene>. The binding site is located on the distal side of heme group IV where <scene name='71/716635/Active_site_1/1'>lysine residues 41, 42, 46 and 50</scene> are an interactive component of the active site. The net charge of these residues is positive, thus binding to negatively charge compounds or cations<ref name="assfalt" />. | ||
== Function == | == Function == | ||
The function of Cc7 was determined by using NMR spectroscopy as a | The function of Cc7 was determined by using NMR spectroscopy as a monitoring technique to determine the oxidation levels of the protein as more chromate (-2)<ref name="assfalt" />. Chromate (-2) nor chromium are part of Cc7's structure; they are the reactant and the product of the reductase activity of Cc7, respectively. The following steps will elaborate how Cc7 carries out its oxidation. | ||
As mentioned previously, Cc7 is a sulfur/metal terminal reductase, an enzyme that reduces a sulfur-containing compounds into a sulfide or reduces heavy metals to generate electrons to be used in electron transport chain<ref | As mentioned previously, Cc7 is a sulfur/metal terminal reductase, an enzyme that reduces a sulfur-containing compounds into a sulfide or reduces heavy metals to generate electrons to be used in electron transport chain<ref name="assfalt" />. | ||
To begin the reaction, Cc7 must be in its resting state (i.e. fully reduced). A negatively charged chromate (-2) interacts and binds with the positively charged surface area of Cc7 containing positive lysine residues located on the distal side of heme IV. Lysine residues 41, 42, 46 and 50 form the binding site for the chromate (-2) ion. The first round of reductions takes place once the chromate (-2) ion binds to the active site. Binding of the chromate (-2) ion induces the iron (II) atom of heme groups I and III to be oxidized first. Because of the close proximity of all the heme groups, the oxidation of heme groups I and III causes heme group IV to reduce the chromate (-2) ion | To begin the reaction, Cc7 must be in its resting state (i.e. fully reduced). A negatively charged chromate (-2) interacts and binds with the positively charged surface area of Cc7 containing the positive lysine residues located on the distal side of heme IV. Lysine residues 41, 42, 46 and 50 form the binding site for the chromate (-2) ion. The first round of reductions takes place once the chromate (-2) ion binds to the active site. Binding of the chromate (-2) ion induces the iron (II) atom of heme groups I and III to be oxidized first. Because of the close proximity of all the heme groups, the oxidation of heme groups I and III causes heme group IV to reduce the chromate (-2) ion. | ||
[[Image:Tileshop.fcgi.jpg|400px|alt=Alt text|Figure 1<ref name="assfalt" />: The binding of chromate (-2) to the active site causes a conformational change in Cc7, resulting in the repositioning of heme groups I, III, and IV which induces reductase enzymatic activity.]] | |||
= | To put it simply, the heme groups I and III push the electron from heme group IV into the chromate (-2) ion. The process continues sequentially from heme III to heme I as each electron from their iron (II) are donated to chromium atom via heme IV. This ability is called '''electron tunnelling'''<ref name="assfalt" /> and it believed to be the main driving force for this reaction to occur. This is thought to be possible as heme groups I and III are able to re-establish thermodynamic conditions by transferring their free iron (II) electron to heme IV. The final result of this series of reductions is a chromium (III) ion. The chromium (III) ion remains in the same region where the charged chromate (-2) ion was first bound. This is made possible by the positive lysine residues present in the active site. The positive nature of the lysine side chains binds the cation in place. | ||
Therefore, the final products of the reaction are a chromium (III) ion and the oxidized Cc7 with three iron(III) hemes. | |||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Latest revision as of 16:49, 6 November 2019
GeneralGeneral
Cytocrhome c 7 (Cc7) is a three heme-containing protein derived from the sulfur-reducing bacterium Desulfuromonas acetoxidans. Cc7 is crucial to the bacteria's anaerobic sulfur respiration as it plays a role in the electron-transfer mechanism, and as such it is located in the mitochondrial inter-membrane space[1]. Over the course of 3.5 million years, Desulfuromonas acetoxidans have evolved to use anaerobic sulfur respiration as the main driving force of their survival[2]. As a result, Cc7 is able to reduce sulfur, its oxidized variants (thiosulfate, sulfite, sulfate, etc) and even heavy metals to produce the required energy[3]. If using sulfur, the product formed is hydrogen sulfide. This compound is able to react with heavy metal ions to form almost non-lethal and quite insoluble metal sulfides[1]. This enzymatic ability of Cc7 is unique to the animal kingdom, and the ability to create less-toxic and insoluble metal sulfides has intrigued researchers as of lateCite error: Closing Structural ComponentsCc7 is a single polypeptide chain 68 residues total containing three heme groups. The polypeptide strand has one alpha helix 4 residues in length and two beta strands 2 residues in length. The heme groups are labelled . The binding site is located on the distal side of heme group IV where are an interactive component of the active site. The net charge of these residues is positive, thus binding to negatively charge compounds or cations[1]. FunctionThe function of Cc7 was determined by using NMR spectroscopy as a monitoring technique to determine the oxidation levels of the protein as more chromate (-2)[1]. Chromate (-2) nor chromium are part of Cc7's structure; they are the reactant and the product of the reductase activity of Cc7, respectively. The following steps will elaborate how Cc7 carries out its oxidation. As mentioned previously, Cc7 is a sulfur/metal terminal reductase, an enzyme that reduces a sulfur-containing compounds into a sulfide or reduces heavy metals to generate electrons to be used in electron transport chain[1]. To begin the reaction, Cc7 must be in its resting state (i.e. fully reduced). A negatively charged chromate (-2) interacts and binds with the positively charged surface area of Cc7 containing the positive lysine residues located on the distal side of heme IV. Lysine residues 41, 42, 46 and 50 form the binding site for the chromate (-2) ion. The first round of reductions takes place once the chromate (-2) ion binds to the active site. Binding of the chromate (-2) ion induces the iron (II) atom of heme groups I and III to be oxidized first. Because of the close proximity of all the heme groups, the oxidation of heme groups I and III causes heme group IV to reduce the chromate (-2) ion. To put it simply, the heme groups I and III push the electron from heme group IV into the chromate (-2) ion. The process continues sequentially from heme III to heme I as each electron from their iron (II) are donated to chromium atom via heme IV. This ability is called electron tunnelling[1] and it believed to be the main driving force for this reaction to occur. This is thought to be possible as heme groups I and III are able to re-establish thermodynamic conditions by transferring their free iron (II) electron to heme IV. The final result of this series of reductions is a chromium (III) ion. The chromium (III) ion remains in the same region where the charged chromate (-2) ion was first bound. This is made possible by the positive lysine residues present in the active site. The positive nature of the lysine side chains binds the cation in place. Therefore, the final products of the reaction are a chromium (III) ion and the oxidized Cc7 with three iron(III) hemes.
|
| ||||||||||
ReferencesReferences
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Assfalg M, Bertini I, Bruschi M, Michel C, Turano P. The metal reductase activity of some multiheme cytochromes c: NMR structural characterization of the reduction of chromium(VI) to chromium(III) by cytochrome c(7). 2002; 99(15):9750-4 DOI: 10.1073/pnas.152290999
- ↑ Barton L, Fauque G. Biochemistry, Physiology and Biotechnology of Sulfate-Reducing Bacteria. Advances in Applied Microbiology. 2009; 68: 41–98. DOI: 10.1016/s0065-2164(09)01202-7
- ↑ Pfennig N, Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. 1976; 110(1): 3-12 DOI: 10.1007/BF00416962