Growth factor receptor-bound protein: Difference between revisions
Jump to navigation
Jump to search
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (23 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
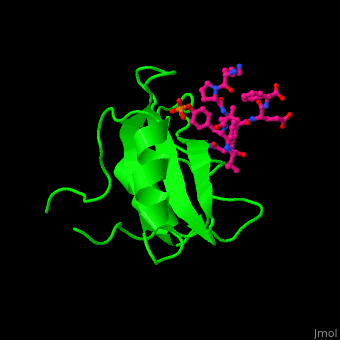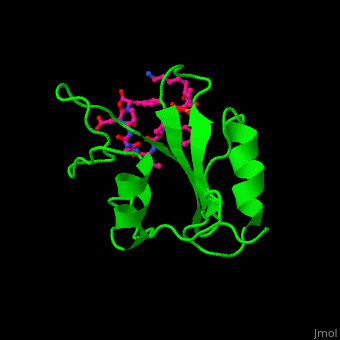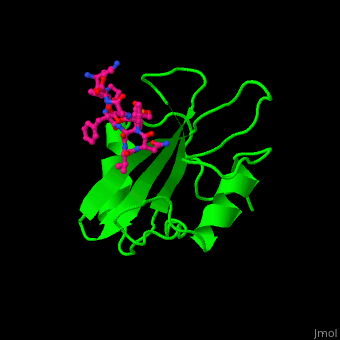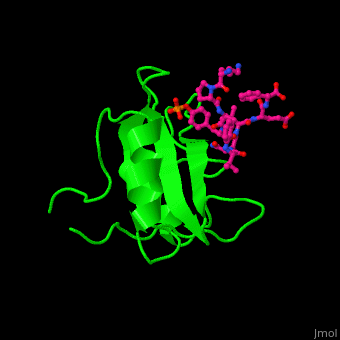
<StructureSection load='' size='350' side='right' caption='Structure of human Grb2 SH2 domain (green) complex with phosphotyrosyl polypeptide (magenta) (PDB entry [[1bmb]])' scene='51/516448/Cv/1'> | |||
<StructureSection load=' | == Function == | ||
'''Growth factor receptor-bound proteins''' (GRB) are adaptor proteins. GRBs contain SH2, Ras-associated (RA) and pleckstrin homology (PH) domains. <br /> | |||
'''Growth factor receptor-bound proteins''' (GRB) are adaptor proteins. '''GRB2''' links the epidermal growth factor receptor tyrosine kinase to the activation of Ras and its downstream kinases. GRB2 contains an SH2 domain which binds tyrosine phosphorylated sequences and 2 SH3 domains which direct complex formation of proline-rich sequences. GRB2 is involved in signal transduction. '''GRB7''' interacts with EGFR and ephrin receptors and plays a role in itegrin signalling pathway. '''GRB10''' | * '''GRB2''' links the epidermal growth factor receptor tyrosine kinase to the activation of Ras and its downstream kinases. GRB2 contains an SH2 domain which binds tyrosine phosphorylated sequences and 2 SH3 domains which direct complex formation of proline-rich sequences. GRB2 is involved in signal transduction<ref>PMID:1322798</ref>.<br /> | ||
* '''GRB7''' interacts with EGFR and ephrin receptors and plays a role in itegrin signalling pathway<ref>PMID:19717535</ref>.<br /> | |||
* '''GRB10''' and '''GRB14''' interact with insulin receptor and insulin-like growth factor receptors<ref>PMID:12783867</ref>. For additional details on GRB10 see [[Grb10 SH2 Domain]].<br /> | |||
== Structural highlights == | |||
Human GRB2 residue <scene name='51/516448/Cv/5'>Trp121 forces the phosphotyrosyl containing ligand</scene> into a turn conformation. Water molecules are shown as red spheres. <scene name='51/516448/Cv/6'>Phosphotyrosine binding</scene> <ref>PMID:10090780</ref>. | |||
==3D structures of growth factor receptor-bound proteins== | |||
[[Growth factor receptor-bound proteins 3D structures]] | |||
</StructureSection> | |||
== References == | |||
<references/> | |||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Latest revision as of 10:53, 18 July 2019
FunctionGrowth factor receptor-bound proteins (GRB) are adaptor proteins. GRBs contain SH2, Ras-associated (RA) and pleckstrin homology (PH) domains.
Structural highlightsHuman GRB2 residue into a turn conformation. Water molecules are shown as red spheres. [4]. 3D structures of growth factor receptor-bound proteinsGrowth factor receptor-bound proteins 3D structures
|
| ||||||||||
ReferencesReferences
- ↑ Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431-42. PMID:1322798
- ↑ Nadler Y, Gonzalez AM, Camp RL, Rimm DL, Kluger HM, Kluger Y. Growth factor receptor-bound protein-7 (Grb7) as a prognostic marker and therapeutic target in breast cancer. Ann Oncol. 2010 Mar;21(3):466-73. doi: 10.1093/annonc/mdp346. Epub 2009 Aug 28. PMID:19717535 doi:http://dx.doi.org/10.1093/annonc/mdp346
- ↑ Deng Y, Bhattacharya S, Swamy OR, Tandon R, Wang Y, Janda R, Riedel H. Growth factor receptor-binding protein 10 (Grb10) as a partner of phosphatidylinositol 3-kinase in metabolic insulin action. J Biol Chem. 2003 Oct 10;278(41):39311-22. Epub 2003 Jun 3. PMID:12783867 doi:http://dx.doi.org/10.1074/jbc.M304599200
- ↑ Ettmayer P, France D, Gounarides J, Jarosinski M, Martin MS, Rondeau JM, Sabio M, Topiol S, Weidmann B, Zurini M, Bair KW. Structural and conformational requirements for high-affinity binding to the SH2 domain of Grb2(1). J Med Chem. 1999 Mar 25;42(6):971-80. PMID:10090780 doi:10.1021/jm9811007