Cholera toxin: Difference between revisions
Michal Harel (talk | contribs) No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| (13 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
<StructureSection load='1xtc' size='400' side='right' scene='43/430104/Cv/2' caption='Cholera toxin composed of A1 (grey), A2 (pink) subunit and a pentamer of B subunits (rust, cyan, gold, yellow, purple). [[1xtc]]'> | |||
[[Cholera toxin]] (CTX), secreted by bacterium [http://en.wikipedia.org/wiki/Vibrio_cholerae Vibrio Cholerae],is an oligomeric complex of enzymatic A and pentameric B subunits, which bind to the cell surface. CTX is the main cause of the diarrhea symptoms of cholera infection. | [[Cholera toxin]] (CTX), secreted by bacterium [http://en.wikipedia.org/wiki/Vibrio_cholerae Vibrio Cholerae],is an oligomeric complex of enzymatic A and pentameric B subunits, which bind to the cell surface. CTX is the main cause of the diarrhea symptoms of cholera infection. | ||
== Structure == | == Structure == | ||
[[Image:CTX interaction.PNG|left|270px|thumb| Interaction of 7 chains of Cholera toxin[[1xtc]]. Cholera toxin contains 7 chains: A,C,D,E,F,G and H.Chains A and C belong to subunit A. Chains | [[Image:CTX interaction.PNG|left|270px|thumb| Interaction of 7 chains of Cholera toxin [[1xtc]]. Cholera toxin contains 7 chains: A,C,D,E,F,G and H.Chains A and C belong to subunit A. Chains D,E,F,G and H belong to subunit B]] | ||
Cholera toxin(CTX) has two types of subunits: subunit A and subunit B. A subunit contains A1 domain, which includes the enzymatic active site, and A2 domain, which has a α–helix tail. The B subunit contains five chains that form a pentameric ring around the central pore in structure; Subunit A and subunit B are assembled by the α–helix tail of A2 domain, which inserts into the central pore. CTX is the main virulence factor of the pathogen ''Vibrio cholerae'' and cause the major symptom of infection: extreme diarrhea, vomiting, cramps and even death <ref>Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. p. 375. ISBN 0838585299.</ref>,<ref>Faruque SM; Nair GB (editors). (2008). Vibrio cholerae: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-33-2.</ref>,<ref>Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro</ref>. | {{Clear}} | ||
Cholera toxin (CTX) has two types of subunits: subunit A and subunit B. <scene name='43/430104/Cv/3'>A subunit</scene> contains A1 domain, which includes the enzymatic active site, and A2 domain, which has a α–helix tail. The <scene name='43/430104/Cv/4'>B subunit</scene> contains <scene name='43/430104/Cv/5'>five chains that form a pentameric ring around the central pore</scene> in structure; Subunit A and subunit B are assembled by the α–helix tail of A2 domain, which inserts into the central pore. CTX is the main virulence factor of the pathogen ''Vibrio cholerae'' and cause the major symptom of infection: extreme diarrhea, vomiting, cramps and even death <ref>Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. p. 375. ISBN 0838585299.</ref>,<ref>Faruque SM; Nair GB (editors). (2008). Vibrio cholerae: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-33-2.</ref>,<ref>Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro</ref>. | |||
The enzymatic subunit has a globular domain (CTA1) and a long helical domain (CTA2). Once the CTX binds to the cell surface, it is internalized, and its CTA1 domain binds to ADP-ribosylation factor 6(Arf6)[http://en.wikipedia.org/wiki/ADP_ribosylation_factor], enabling its catalytic activity. | The enzymatic subunit has a globular domain (CTA1) and a long helical domain (CTA2). Once the CTX binds to the cell surface, it is internalized, and its CTA1 domain binds to ADP-ribosylation factor 6(Arf6)[http://en.wikipedia.org/wiki/ADP_ribosylation_factor], enabling its catalytic activity. | ||
{{Clear}} | |||
== mRNA Structure == | == mRNA Structure == | ||
[[Image:CTX_RNA.png|left|280px|thumb|mRNA structure of CTX. The structure is predicted by the method of minimum free energy, using RNAfold WebServer.The colors represent the propensity of each nucleotide to participate in base pairs and whether a predicted base pair is well predicted. The scale ranges from red (highest probability) to blue-violet (lower probability).]] | [[Image:CTX_RNA.png|left|280px|thumb|mRNA structure of CTX. The structure is predicted by the method of minimum free energy, using RNAfold WebServer.The colors represent the propensity of each nucleotide to participate in base pairs and whether a predicted base pair is well predicted. The scale ranges from red (highest probability) to blue-violet (lower probability).]] | ||
| Line 23: | Line 17: | ||
== Function == | == Function == | ||
Cholera toxin, after being secreted from the ''Vibrio cholerae'', binds to the enterocytes (intestinal cells) by the interaction between the subunit B and GM1 ganglioside[http://en.wikipedia.org/wiki/GM1] receptor on enterocytes, which then promotes the toxin endocytosis. Next, A1 turns to an active enzyme after separating with the A2 domain. After the A1 domain of subunit A of the toxin enters the cytosol, it activates adenylate cyclase to produce cAMP through G proteins, which triggers the activation of cystic fibrosis transmembrane conductance regulator (CFTR), leading to watery diarrhea: the efflux of water and ions from cells <ref>Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro</ref>. | Cholera toxin, after being secreted from the ''Vibrio cholerae'', binds to the enterocytes (intestinal cells) by the interaction between the subunit B and GM1 ganglioside[http://en.wikipedia.org/wiki/GM1] receptor on enterocytes, which then promotes the toxin endocytosis. Next, A1 turns to an active enzyme after separating with the A2 domain. After the A1 domain of subunit A of the toxin enters the cytosol, it activates adenylate cyclase to produce cAMP through G proteins, which triggers the activation of cystic fibrosis transmembrane conductance regulator (CFTR), leading to watery diarrhea: the efflux of water and ions from cells <ref>Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro</ref>. | ||
[[Image:Picture1.png|left|300px|thumb|Structure Notation of the RNA of CTX A, CLC RNA Workbench]] | |||
{{Clear}} | |||
== Evolution == | == Evolution == | ||
CTXφ Bacteriophage which is carried by ''Vibrio cholerae'' produces Cholera toxin. Cholera toxin is encoded by the ctxA and ctxB genes that are introduced into V. cholerae strains by horizontal gene transfer <ref>Davis B, Waldor M (2003). "Filamentous phages linked to virulence of Vibrio cholerae". Curr Opin Microbiol 6 (1): 35–42. doi:10.1016/S1369-5274(02)00005-X. PMID 12615217.</ref>. | CTXφ Bacteriophage which is carried by ''Vibrio cholerae'' produces Cholera toxin. Cholera toxin is encoded by the ctxA and ctxB genes that are introduced into V. cholerae strains by horizontal gene transfer <ref>Davis B, Waldor M (2003). "Filamentous phages linked to virulence of Vibrio cholerae". Curr Opin Microbiol 6 (1): 35–42. doi:10.1016/S1369-5274(02)00005-X. PMID 12615217.</ref>. | ||
| Line 29: | Line 24: | ||
== Application == | == Application == | ||
Subunit B of cholera toxin is designed to be applied as a neuronal tracer due to its non-toxic characteristic. It also used to identify lipid rafts as florescent tag on the cell surface since lipid rafts contains GM1 gangliosides, which will interact with subunit B during mechanism <ref>Luppi P.H.. "The Discovery of Cholera-Toxin as a Powerful Neuroanatomical Tool". Retrieved 2011-03-23.</ref>. It is demonstrated that cholera toxin subunit B is a sensitive retrograde tracer for the central nervous system <ref>Luppi P.H., Fort P., Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 534 (1-2) pages : 209-224 (1990)</ref>. | Subunit B of cholera toxin is designed to be applied as a neuronal tracer due to its non-toxic characteristic. It also used to identify lipid rafts as florescent tag on the cell surface since lipid rafts contains GM1 gangliosides, which will interact with subunit B during mechanism <ref>Luppi P.H.. "The Discovery of Cholera-Toxin as a Powerful Neuroanatomical Tool". Retrieved 2011-03-23.</ref>. It is demonstrated that cholera toxin subunit B is a sensitive retrograde tracer for the central nervous system <ref>Luppi P.H., Fort P., Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 534 (1-2) pages : 209-224 (1990)</ref>. | ||
== Multiple Sequence Alignment == | == Multiple Sequence Alignment == | ||
[[Image:MSA1.jpg|left| | [[Image:MSA1.jpg|left|300px|thumb|Multiple Sequence Alignment-TEXSHADE result.BlastP of CTX A subunit that contains six different species. This is made by Biology Workbench]] | ||
{{Clear}} | |||
'''Selected Sequences:''' | '''Selected Sequences:''' | ||
| Line 73: | Line 41: | ||
6. Vibrio cholera O1 str. 2010EL-1786 chromosome 1, complete | 6. Vibrio cholera O1 str. 2010EL-1786 chromosome 1, complete | ||
[[Image:TREE - MAXIMUM LIKEHOOD.PNG|left|430px|thumb|Phylogenetic Tree of MSA of CTX, Statistic Method:Maximum Likelihood, made by MEGA 5.05]] | |||
== 3D Structures of Cholera toxin == | |||
[[Cholera toxin 3D structures]] | |||
</StructureSection> | |||
< | |||
== External Links == | == External Links == | ||
PDB ID: 1XTC [http://www.rcsb.org/pdb/101/motm_disscussed_entry.do?id=1xtc] | PDB ID: 1XTC [http://www.rcsb.org/pdb/101/motm_disscussed_entry.do?id=1xtc] | ||
| Line 84: | Line 53: | ||
PubMed[http://www.ncbi.nlm.nih.gov/pubmed/7658473?dopt=Abstract] | PubMed[http://www.ncbi.nlm.nih.gov/pubmed/7658473?dopt=Abstract] | ||
== See also [[Toxins]] == | |||
== Reference == | |||
<references /> | |||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Latest revision as of 12:44, 12 May 2019
Cholera toxin (CTX), secreted by bacterium Vibrio Cholerae,is an oligomeric complex of enzymatic A and pentameric B subunits, which bind to the cell surface. CTX is the main cause of the diarrhea symptoms of cholera infection. Structure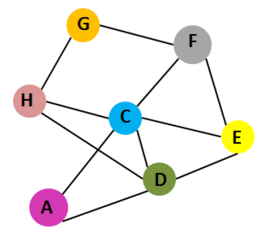 Cholera toxin (CTX) has two types of subunits: subunit A and subunit B. contains A1 domain, which includes the enzymatic active site, and A2 domain, which has a α–helix tail. The contains in structure; Subunit A and subunit B are assembled by the α–helix tail of A2 domain, which inserts into the central pore. CTX is the main virulence factor of the pathogen Vibrio cholerae and cause the major symptom of infection: extreme diarrhea, vomiting, cramps and even death [1],[2],[3]. The enzymatic subunit has a globular domain (CTA1) and a long helical domain (CTA2). Once the CTX binds to the cell surface, it is internalized, and its CTA1 domain binds to ADP-ribosylation factor 6(Arf6)[1], enabling its catalytic activity. mRNA Structure RNA folding algorithm uses a method to minimize the free energy of the structure so that the RNA molecule is in the most stable form. The image at lower left is the predicted mRNA structure of CTX, which is made by RNAfold WebServer. FunctionCholera toxin, after being secreted from the Vibrio cholerae, binds to the enterocytes (intestinal cells) by the interaction between the subunit B and GM1 ganglioside[2] receptor on enterocytes, which then promotes the toxin endocytosis. Next, A1 turns to an active enzyme after separating with the A2 domain. After the A1 domain of subunit A of the toxin enters the cytosol, it activates adenylate cyclase to produce cAMP through G proteins, which triggers the activation of cystic fibrosis transmembrane conductance regulator (CFTR), leading to watery diarrhea: the efflux of water and ions from cells [4].  EvolutionCTXφ Bacteriophage which is carried by Vibrio cholerae produces Cholera toxin. Cholera toxin is encoded by the ctxA and ctxB genes that are introduced into V. cholerae strains by horizontal gene transfer [5]. ApplicationSubunit B of cholera toxin is designed to be applied as a neuronal tracer due to its non-toxic characteristic. It also used to identify lipid rafts as florescent tag on the cell surface since lipid rafts contains GM1 gangliosides, which will interact with subunit B during mechanism [6]. It is demonstrated that cholera toxin subunit B is a sensitive retrograde tracer for the central nervous system [7]. Multiple Sequence Alignment Selected Sequences: 1. Cholera toxin Subunit A 2. Escherichia coli E24377A plasmid pETEC_80, complete sequence 3. Escherichia coli strain 214-III elt operon, complete sequence 4. Vibrio cholerae strain JS9803 prophage Vibrio phage CTX Zot (zot) 5. Vibrio cholera O395 chromosome II, complete sequence 6. Vibrio cholera O1 str. 2010EL-1786 chromosome 1, complete  3D Structures of Cholera toxin
|
| ||||||||||
External LinksExternal Links
PDB ID: 1XTC [1]
MMDB ID: 52036 [2]
PubMed[3]
See also ToxinsSee also Toxins
ReferenceReference
- ↑ Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. p. 375. ISBN 0838585299.
- ↑ Faruque SM; Nair GB (editors). (2008). Vibrio cholerae: Genomics and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-33-2.
- ↑ Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro
- ↑ Jennifer McDowall, Cholera Toxin, EMBL-EMI, Interpro
- ↑ Davis B, Waldor M (2003). "Filamentous phages linked to virulence of Vibrio cholerae". Curr Opin Microbiol 6 (1): 35–42. doi:10.1016/S1369-5274(02)00005-X. PMID 12615217.
- ↑ Luppi P.H.. "The Discovery of Cholera-Toxin as a Powerful Neuroanatomical Tool". Retrieved 2011-03-23.
- ↑ Luppi P.H., Fort P., Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 534 (1-2) pages : 209-224 (1990)