User:Cody J Cubbage: Difference between revisions
No edit summary |
No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
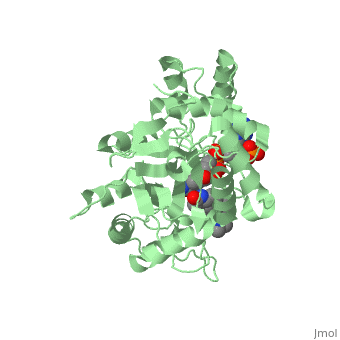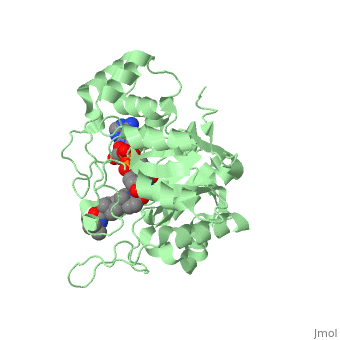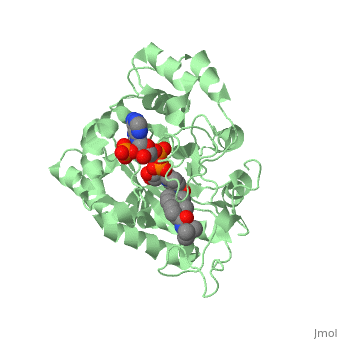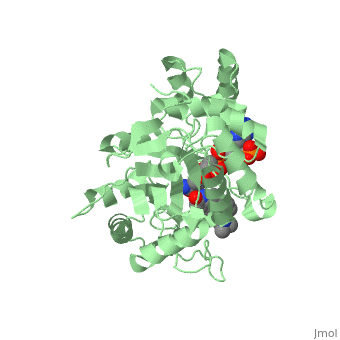
<StructureSection load='3G1R' size='350' side='right' scene='' caption='N-(1,1-dimethylethyl)-3-oxo- | <StructureSection load='3G1R' size='350' side='right' scene='' caption='N-(1,1-dimethylethyl)-3-oxo- | ||
(5α,17β)-4-azaandrost-1-ene-17-carboxamide bound to | (5α,17β)-4-azaandrost-1-ene-17-carboxamide bound to 5β-reductase (PDB code [[3g1r]])'> | ||
==Function== | ==Function== | ||
Finasteride, branded as Proscar or Propecia, is a synthetic 4-azasteroid compound that acts as a 5α-reductase inhibitor.<ref name="one"> I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1 </ref> The 5α-reductase enzyme is very important in the metabolism of many of the steroids produced by the body, in particular the conversion of testosterone to dihydrotestosterone (DHT).<ref name="one"> I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1 </ref> For this reason, Finasteride is used as a treatment for benign prostate hyperplasia (BPH), which is caused by an overproduction of DHT in the male prostate.<ref name="two"> Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035 </ref> | Finasteride, branded as Proscar or Propecia, is a synthetic 4-azasteroid compound that acts as a 5α-reductase inhibitor.<ref name="one"> I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1 </ref> The 5α-reductase enzyme is very important in the metabolism of many of the steroids produced by the body, in particular the conversion of testosterone to dihydrotestosterone (DHT).<ref name="one"> I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1 </ref> For this reason, Finasteride is used as a treatment for benign prostate hyperplasia (BPH), which is caused by an overproduction of DHT in the male prostate.<ref name="two"> Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035 </ref> Androgenetic Alopecia or male pattern baldness (MPB) is another condition in men caused by the build up of DHT, which can also be treated with Finasteride.<ref name="three"> Varothai, S; Bergfeld, WF (Jul 2014). "Androgenetic alopecia: an evidence-based treatment update.". American journal of clinical dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508 </ref> Finasteride's affinity for 5α-reductase approaches that of testosterone, and can bind to two of the three isoenzymes of 5α-reductase, types I and II.<ref name="two"> Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035 </ref> | ||
==Structure== | ==Structure== | ||
To see what Finasteride looks like unbound, click this link: <scene name='74/745970/Finasteride_unbound/1'>FIT</scene>. Finasteride belongs to the subclass of steroids called azasteroids. All steroids contain a core structure called a gonane, a structure composed of 3 cyclohexane rings and 1 cyclopentane ring "fused" together.<ref name="ten"> Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3 </ref> Although a gonane has 6 chirality centers, giving it 64 possible steroisomers, the majority of steroids contain the 5α-gonane stereoisomer. <ref name="eleven"> Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9 </ref> The function and naming of steroids are determined by what functional groups are attached to the rings of gonane and by the substitution of carbon atoms in the rings with different atoms. In the case of Finasteride, two methyl groups have been attached to carbons 10 and 13 of the gonane structure, placing it in the androstane classification of steroids.<ref name="ten"> Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3 </ref> The gonane ring is further modified by the addition of a double bond at carbon 1 and a ketone to carbon 3. Carbon 4 in Finasteride has been substituted with a nitrogen atom, making Finasteride a 4-azasteroid. Finally, a carboxamide with a ''tert''-butyl group bonded to the nitrogen atom, has been added onto the carbon 17. | To see what Finasteride looks like unbound, click this link: <scene name='74/745970/Finasteride_unbound/1'>FIT</scene>. Finasteride belongs to the subclass of steroids called azasteroids. All steroids contain a core structure called a gonane, a structure composed of 3 cyclohexane rings and 1 cyclopentane ring "fused" together.<ref name="ten"> Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3 </ref> Although a gonane has 6 chirality centers, giving it 64 possible steroisomers, the majority of steroids contain the 5α-gonane stereoisomer. <ref name="eleven"> Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9 </ref> The function and naming of steroids are determined by what functional groups are attached to the rings of gonane and by the substitution of carbon atoms in the rings with different atoms. In the case of Finasteride, two methyl groups have been attached to carbons 10 and 13 of the gonane structure, placing it in the androstane classification of steroids.<ref name="ten"> Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3 </ref> The gonane ring is further modified by the addition of a double bond at carbon 1 and a ketone to carbon 3. Carbon 4 in Finasteride has been substituted with a nitrogen atom, making Finasteride a 4-azasteroid. Finally, a carboxamide with a ''tert''-butyl group bonded to the nitrogen atom, has been added onto the carbon 17 (Figure 1). | ||
There is no known three-dimensional structure of 5α-redcutase to show how it binds with Finasteride. However, there is a known structure for the enzyme 5β-reductase bound to Finasteride and NADP by a mechanism that is similar to the binding of 5α-reductase to Finasteride. The interactions between 5β-reductase and Finasteride and NADP were determined by using the homology modeling software Yasara and Uniprot that provided an accessible resource of protein sequence and functional information (Figure 2). | |||
[[Image: Finasteride.PNG|thumb|300px|left|'''Fig. 1'''. Structure of Finasteride.]] | [[Image: Finasteride.PNG|thumb|300px|left|'''Fig. 1'''. Structure of Finasteride.]] | ||
[[Image: Capture.JPG|thumb|300px|right|'''Fig. 1'''The interaction between 5β-reductase (green) and Finasteride (gray) and NADP (blue). Two Tyrosine (58 and 132 in yellow), two Tryptophan (89 and 230 in red) and Glutamic acid (120 in orange) residues in 5β-reductase are interacting with Finasteride. While Glutamine (193 in light blue) and aspartic acid (53 in purple) residues in 5β-reductase are interacting with NADP.]] | |||
{{Clear}} | {{Clear}} | ||
==Mechanism== | ==Mechanism== | ||
Finasteride is a | Finasteride is a 5α-reductase inhibitor. There are three isoforms of 5α-reductase, types I, II, and III. While the drug has a higher affinity for the type II enzyme, it also inhibits the function of the type I and no affinity for type III.<ref name="four"> Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm </ref>The enzyme 5α-reductase turns Finasteride into dihydrofinasteride through an enzyme bound, NADP-dihydrofinasteride adduct.. However, Finasteride , having a high affinity for 5α-reductase, covalently binds to the enzyme as a Michael acceptor, through a functionally irreversible reaction which essentially halts the chemical process before the NADP-Dihydrofinasteride complex can be reduced to Dihydrofinasteride because of a change in the carbanion position in respect to its placement on the similar enolate intermediate created in the reaction where testosterone is converted into DHT by 5α-reductase.(figure 3) However, the NADP-dihydrofinasteride complex does eventually break down with a half life of about 1 month at 37˚C., which is why patients must continue taking the drug.<ref name="five"> Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t</ref> | ||
[[Image: Biochem group project.PNG|thumb|500px|right|'''Fig. | [[Image: Biochem group project.PNG|thumb|500px|right|'''Fig. 3'''. Proposed mechanism of inhibition by Bull et. al. Image shows how the inhibition of 5alpha-reductase is achieved as Finasteride is used as a substrate to create an enolate intermediate, similar to the one made during the reduction of testosterone. However, the complex created does not allow for the proton transfer needed to complete the reduction of NADP-Dihydrofinasteride to Finasteride, because of the change in the carbanion position. PADPR= phosphoadenosine diphosphoribose<ref name="five">Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. </ref>]] | ||
; | |||
==Medical Uses== | ==Medical Uses== | ||
==='''Benign Prostatic Hyperplasia (BPH)'''=== | |||
[[Aromatase]] and 5α-reductase is responsible for converting androgen hormones into estrogen and dihydrotestosterone (DHT). This chemical process of androgen hormones leads to a decrease in testosterone, but raises levels of DHT and estrogen <ref name="eight">Tacklind, J., Fink, H.A., MacDonald, R., Rutks, I., Wilt, T.J. (2010). Finasteride for benign prostatic hyperplasia. Cochrane database of systematic reviews 2010, Issue 10. Art. No.: CD006015. DOI: 10.1002/14651858.CD006015.pub3. </ref>. Estrogen is a key role in cells proliferating in the prostate and DHT is an anabolic hormone much more potent (dissociated from the androgen receptor slowly) than testosterone that when combined, causes a synergy to induce BPH <ref name="nine">Dragan, I., Misso, M. (2012). Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: A systematic review. Maturitas, 72 (4), 269 </ref>. The enzyme 5α-reductase is responsible for the synthesis of DHT in the prostate from circulating testosterone. 5α-reductase is located in the stromal cells, which is the main site for the synthesis of DHT, but it can also diffuse into epithelial cells close-by. In both stromal and epithelial cells, DHT binds to nuclear androgen receptors and signals for transcription for cell growth. Finasteride is used to inhibit the 5α-reductase enzyme, which blocks the conversion of testosterone and inhibits the production of DHT, reducing prostate volume and BPH symptoms (urinating complication). Using finasteride could increase the risk for erectile dysfunction, decrease libido, and ejaulation disorder due to 5α-reductase being inhibited. | |||
==='''Prostate Cancer'''=== | |||
Prostate cancer is an androgen dependant adenocarcinoma. The FDA has not approved finasteride as a treatment for prostate cancer. A warning was added, 5α-reductase inhibitors concerning an increased risk of high-grade prostate cancer, as the treatment of BPH lowers PSA (prostate-specific antigen), which could mask that prostate cancer is present. | |||
==='''Androgenetic Alopecia (AGA)'''=== | |||
Androgenetic alopecia (AGA) is an androgen-dependent, progressively thinning of the scalp hair. AGA is also referred to as male-pattern hair loss or baldness (MPB) and female-pattern hair loss in women. It is characterized by progressive shortening of the duration of anagen with successive hair cycles, leading to decreased numbers of hair in anagen at any given time, and aggressive follicular miniaturization with conversion of terminal to vellus-like follicles <ref name="ten"> Paus, R., Cotsarelis, G. (1999).The biology of hair follicles. N. Engl. J. Med., 34, 491–497. </ref> The reason for the use of finasteride to treat AGA in men is based on the absence of AGA in men with congenital deficiency of type 2 5α-reductase, and the presence of increased 5α-reductase activity and DHT levels in balding scalp <ref name="eleven"> Yamana, K., Labrie, F., Luu-The, V. (2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035. </ref>. This condition is caused by a build up of DHT in tissues, which in the case of MPB, is the scalp. The build up of DHT causes androgen-dependent miniaturization of scalp hair follicles. Testosterone is the primary androgen in the body, but to be maximally active in scalp hair follicles it must be converted to dihydrotestosterone (DHT) by the enzyme 5α-reductase. Inhibition of the enzyme with Finasteride has been shown to reduce both serum and scalp skin DHT levels in balding men. In some patients, hair regrowth can occur.<ref name="twelve"> Olsen, E. A., Hordinsky, M., & Whiting, D., et al. (2006, December). </ref> Side effects from Finasteride include but are not limited to, decreased sexual ability and desire. <ref name="thirteen"> Leyden, James et al.(June 1999)."Finasteride in the treatment of men with frontal male pattern hair loss." Journal of the American Academy of Dermatology. Volume 40 , Issue 6 , 930 - 937 </ref> | |||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Latest revision as of 16:38, 6 December 2016
N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide (Finasteride)N-(1,1-dimethylethyl)-3-oxo-(5α,17β)-4-azaandrost-1-ene-17-carboxamide (Finasteride)
FunctionFinasteride, branded as Proscar or Propecia, is a synthetic 4-azasteroid compound that acts as a 5α-reductase inhibitor.[1] The 5α-reductase enzyme is very important in the metabolism of many of the steroids produced by the body, in particular the conversion of testosterone to dihydrotestosterone (DHT).[1] For this reason, Finasteride is used as a treatment for benign prostate hyperplasia (BPH), which is caused by an overproduction of DHT in the male prostate.[2] Androgenetic Alopecia or male pattern baldness (MPB) is another condition in men caused by the build up of DHT, which can also be treated with Finasteride.[3] Finasteride's affinity for 5α-reductase approaches that of testosterone, and can bind to two of the three isoenzymes of 5α-reductase, types I and II.[2] StructureTo see what Finasteride looks like unbound, click this link: . Finasteride belongs to the subclass of steroids called azasteroids. All steroids contain a core structure called a gonane, a structure composed of 3 cyclohexane rings and 1 cyclopentane ring "fused" together.[4] Although a gonane has 6 chirality centers, giving it 64 possible steroisomers, the majority of steroids contain the 5α-gonane stereoisomer. [5] The function and naming of steroids are determined by what functional groups are attached to the rings of gonane and by the substitution of carbon atoms in the rings with different atoms. In the case of Finasteride, two methyl groups have been attached to carbons 10 and 13 of the gonane structure, placing it in the androstane classification of steroids.[4] The gonane ring is further modified by the addition of a double bond at carbon 1 and a ketone to carbon 3. Carbon 4 in Finasteride has been substituted with a nitrogen atom, making Finasteride a 4-azasteroid. Finally, a carboxamide with a tert-butyl group bonded to the nitrogen atom, has been added onto the carbon 17 (Figure 1). There is no known three-dimensional structure of 5α-redcutase to show how it binds with Finasteride. However, there is a known structure for the enzyme 5β-reductase bound to Finasteride and NADP by a mechanism that is similar to the binding of 5α-reductase to Finasteride. The interactions between 5β-reductase and Finasteride and NADP were determined by using the homology modeling software Yasara and Uniprot that provided an accessible resource of protein sequence and functional information (Figure 2).
  MechanismFinasteride is a 5α-reductase inhibitor. There are three isoforms of 5α-reductase, types I, II, and III. While the drug has a higher affinity for the type II enzyme, it also inhibits the function of the type I and no affinity for type III.[6]The enzyme 5α-reductase turns Finasteride into dihydrofinasteride through an enzyme bound, NADP-dihydrofinasteride adduct.. However, Finasteride , having a high affinity for 5α-reductase, covalently binds to the enzyme as a Michael acceptor, through a functionally irreversible reaction which essentially halts the chemical process before the NADP-Dihydrofinasteride complex can be reduced to Dihydrofinasteride because of a change in the carbanion position in respect to its placement on the similar enolate intermediate created in the reaction where testosterone is converted into DHT by 5α-reductase.(figure 3) However, the NADP-dihydrofinasteride complex does eventually break down with a half life of about 1 month at 37˚C., which is why patients must continue taking the drug.[7]  Medical UsesBenign Prostatic Hyperplasia (BPH)Aromatase and 5α-reductase is responsible for converting androgen hormones into estrogen and dihydrotestosterone (DHT). This chemical process of androgen hormones leads to a decrease in testosterone, but raises levels of DHT and estrogen [8]. Estrogen is a key role in cells proliferating in the prostate and DHT is an anabolic hormone much more potent (dissociated from the androgen receptor slowly) than testosterone that when combined, causes a synergy to induce BPH [9]. The enzyme 5α-reductase is responsible for the synthesis of DHT in the prostate from circulating testosterone. 5α-reductase is located in the stromal cells, which is the main site for the synthesis of DHT, but it can also diffuse into epithelial cells close-by. In both stromal and epithelial cells, DHT binds to nuclear androgen receptors and signals for transcription for cell growth. Finasteride is used to inhibit the 5α-reductase enzyme, which blocks the conversion of testosterone and inhibits the production of DHT, reducing prostate volume and BPH symptoms (urinating complication). Using finasteride could increase the risk for erectile dysfunction, decrease libido, and ejaulation disorder due to 5α-reductase being inhibited. Prostate CancerProstate cancer is an androgen dependant adenocarcinoma. The FDA has not approved finasteride as a treatment for prostate cancer. A warning was added, 5α-reductase inhibitors concerning an increased risk of high-grade prostate cancer, as the treatment of BPH lowers PSA (prostate-specific antigen), which could mask that prostate cancer is present. Androgenetic Alopecia (AGA)Androgenetic alopecia (AGA) is an androgen-dependent, progressively thinning of the scalp hair. AGA is also referred to as male-pattern hair loss or baldness (MPB) and female-pattern hair loss in women. It is characterized by progressive shortening of the duration of anagen with successive hair cycles, leading to decreased numbers of hair in anagen at any given time, and aggressive follicular miniaturization with conversion of terminal to vellus-like follicles [4] The reason for the use of finasteride to treat AGA in men is based on the absence of AGA in men with congenital deficiency of type 2 5α-reductase, and the presence of increased 5α-reductase activity and DHT levels in balding scalp [5]. This condition is caused by a build up of DHT in tissues, which in the case of MPB, is the scalp. The build up of DHT causes androgen-dependent miniaturization of scalp hair follicles. Testosterone is the primary androgen in the body, but to be maximally active in scalp hair follicles it must be converted to dihydrotestosterone (DHT) by the enzyme 5α-reductase. Inhibition of the enzyme with Finasteride has been shown to reduce both serum and scalp skin DHT levels in balding men. In some patients, hair regrowth can occur.[10] Side effects from Finasteride include but are not limited to, decreased sexual ability and desire. [11]
|
| ||||||||||
ReferencesReferences
- ↑ 1.0 1.1 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 121, 246. ISBN 978-94-011-4439-1
- ↑ 2.0 2.1 Yamana K, Labrie F, Luu-The V (January 2010). Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035
- ↑ Varothai, S; Bergfeld, WF (Jul 2014). "Androgenetic alopecia: an evidence-based treatment update.". American journal of clinical dermatology. 15 (3): 217–30. doi:10.1007/s40257-014-0077-5. PMID 24848508
- ↑ 4.0 4.1 4.2 Lednicer D (2011). Steroid Chemistry at a Glance. Hoboken: Wiley. ISBN 978-0-470-66084-3 Cite error: Invalid
<ref>tag; name "ten" defined multiple times with different content - ↑ 5.0 5.1 Burkhard Fugmann; Susanne Lang-Fugmann; Wolfgang Steglich (28 May 2014). RÖMPP Encyclopedia Natural Products, 1st Edition, 2000. Thieme. pp. 1918–. ISBN 978-3-13-179551-9 Cite error: Invalid
<ref>tag; name "eleven" defined multiple times with different content - ↑ Schieck, Cynthia L.(1998, August) "Finasteride (Propecia ®)". http://www.chm.bris.ac.uk/motm/finasteride/Finasteride%20(Propecia)%20-%20Feature%20Molecule.htm
- ↑ 7.0 7.1 Bull, Herbert G.*Garcia-Calvo,Margarita Andersson,Stefan†, Baginsky, Walter F.,Chan,H. Karen,Ellsworth,‡ Dina E., Miller,§ Randall R., Stearns,Ralph A.,Bakshi,Raman K.,Rasmusson, Gary H.,Tolman,Richard L., Myers,Robert W.,Kozarich,John W.,Harris,Georgianna S. (1995, August 6) Mechanism-Based Inhibition of Human Steroid 5R-Reductase by Finasteride: Enzyme-Catalyzed Formation of NADP-Dihydrofinasteride, a Potent Bisubstrate Analog Inhibitor. http://pubs.acs.org/doi/pdf/10.1021/ja953069t Cite error: Invalid
<ref>tag; name "five" defined multiple times with different content - ↑ Tacklind, J., Fink, H.A., MacDonald, R., Rutks, I., Wilt, T.J. (2010). Finasteride for benign prostatic hyperplasia. Cochrane database of systematic reviews 2010, Issue 10. Art. No.: CD006015. DOI: 10.1002/14651858.CD006015.pub3.
- ↑ Dragan, I., Misso, M. (2012). Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: A systematic review. Maturitas, 72 (4), 269
- ↑ Olsen, E. A., Hordinsky, M., & Whiting, D., et al. (2006, December).
- ↑ Leyden, James et al.(June 1999)."Finasteride in the treatment of men with frontal male pattern hair loss." Journal of the American Academy of Dermatology. Volume 40 , Issue 6 , 930 - 937