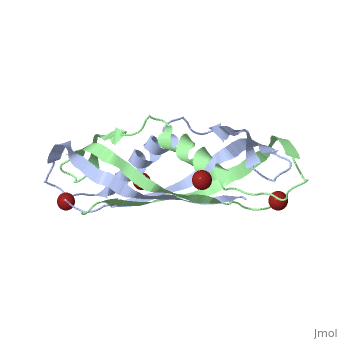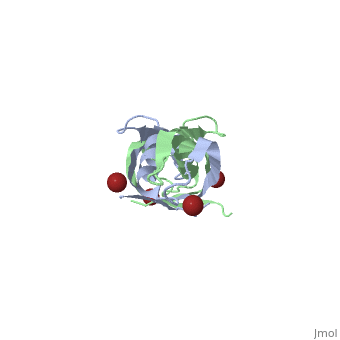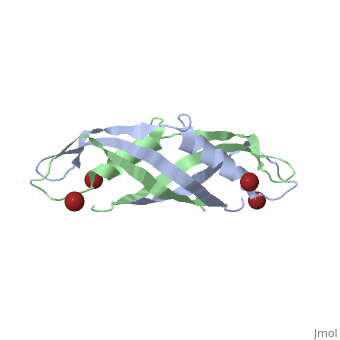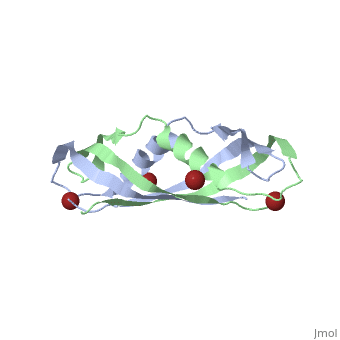
TonB
StructureTonB spans the periplasm [1], with the C-terminus of TonB spanning from residues ~150 to 239[2]. For more details on the Ton system see Ton. FunctionTonB is involved in the uptake of iron as a component of the TonB/ExbB/ExbD complex of the Ton system. It's activity is determined by the presence of ExbB and ExbD[3]. The C-terminus of TonB interacts with outer membrane transporters, known as TonB-dependent outer membrane transporters (TBDTs)[4], allowing the translocation of biochemical molecules between the inner and outer membrane[2]. Interaction with ExbB/ExbDTonB and ExbD bind to ExbB in a protein complex that prevents the degradation of the TonB protein. TonB and ExbD are anchored to the cytoplasmic membrane via their N-terminal hydrophobic sequences, with the rest of the protein complex extending into the periplasmic space, allowing for interaction with outer membrane proteins.[3] TonB takes on the role of an energy transducer, while ExbB and ExbD are involved in the transport of biochemical molecules. In order for sucessful energy transduction to occur, there are processes that must occur[5]:
BtuB-TonB Complex<StructureSection load='2gsk' size='350' side='right' scene='BtuB-TonB_Complex/Btubtonbcomplex/1' caption='E. coli TonB C-terminal (green) complex with vitamin B12 transporter BtuB (grey) (PDB code 2gsk)'> As shown in the 3D structure to the right (2GSK), TonB complexes with BtuB in order to aid the transport of nutrients such as cobalamins[6] across the outer membrane by incorporating the proton-motive force into the outer membrane.[7] TonB attaches to BtuB on the periplasmic side of the 614 amino acid BtuB protein[7]. |
| ||||||||||
3D structures of TonB3D structures of TonB
Updated on 06-December-2020
1ihr, 1qxx, 1u07 – EcTonB C terminal – Escherichia coli
1xx3, 5lw8 - EcTonB C terminal – NMR
2grx - EcTonB + ferrichrome-iron receptor
2ivz - EcTonB + colicin-E9 peptide
2gsk - EcTonB C terminal + vitamin B12 transporter BtuB
2k9k – TonB2 C terminal – Listonella anguillarum
6fip - PaTonB C terminal – Pseudomonas aeruginosa - NMR
6i97 - PaTonB C terminal + TonB-dependent receptor
5lw8, 6sly - TonB C terminal – Helicobacter pylori - NMR
ReferencesReferences
- ↑ Pawelek PD, Croteau N, Ng-Thow-Hing C, Khursigara CM, Moiseeva N, Allaire M, Coulton JW. Structure of TonB in complex with FhuA, E. coli outer membrane receptor. Science. 2006 Jun 2;312(5778):1399-402. PMID:16741125 doi:312/5778/1399
- ↑ 2.0 2.1 Postle K, Kastead KA, Gresock MG, Ghosh J, Swayne CD. The TonB Dimeric Crystal Structures Do Not Exist In Vivo. MBio. 2010 Dec 21;1(5). pii: e00307-10. PMID:21179522 doi:10.1128/mBio.00307-10
- ↑ 3.0 3.1 Kampfenkel K, Braun V. Topology of the ExbB protein in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1993 Mar 15;268(8):6050-7. PMID:8449962
- ↑ Wiener MC. TonB-dependent outer membrane transport: going for Baroque? Curr Opin Struct Biol. 2005 Aug;15(4):394-400. PMID:16039843 doi:10.1016/j.sbi.2005.07.001
- ↑ Held KG, Postle K. ExbB and ExbD do not function independently in TonB-dependent energy transduction. J Bacteriol. 2002 Sep;184(18):5170-3. PMID:12193634
- ↑ Cadieux N, Bradbeer C, Kadner RJ. Sequence changes in the ton box region of BtuB affect its transport activities and interaction with TonB protein. J Bacteriol. 2000 Nov;182(21):5954-61. PMID:11029413
- ↑ 7.0 7.1 Shultis DD, Purdy MD, Banchs CN, Wiener MC. Outer membrane active transport: structure of the BtuB:TonB complex. Science. 2006 Jun 2;312(5778):1396-9. PMID:16741124 doi:312/5778/1396