NalP
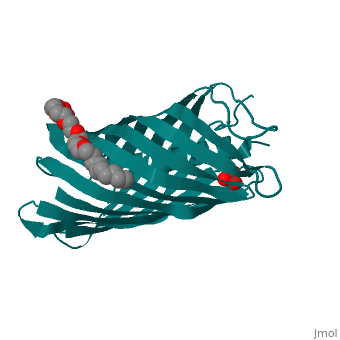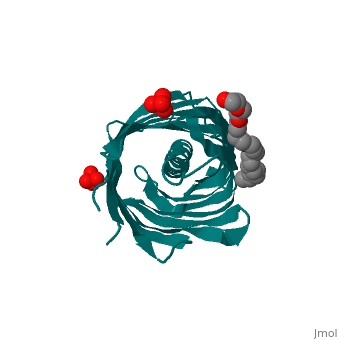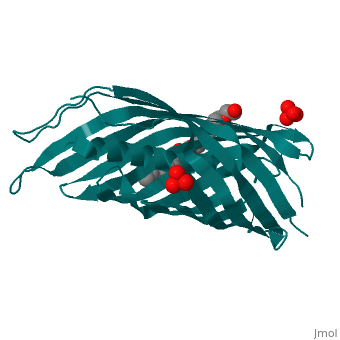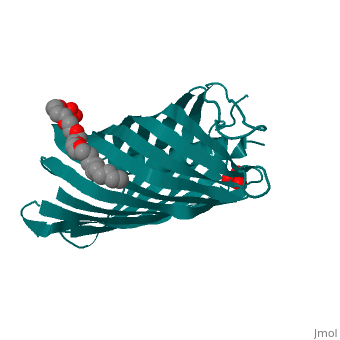
The Translocator Domain for the Autotransporter NaIP within Neisseria meningitidis provides a novel protein pore that contains an alpha helix running axially through its hydrophilic center. Classically many outer membrane pores contain a , which is able to allow for different conditions than the peptidoglycan layer, which would typically stop many types of proteins and ions from passing through. This blocks the pore from being totally open and allows for more regulation of what enters and leaves the cell.[1]
Neisseria meningitidesNeisseria meningitides is a bacterium that infects humans and is the leading cause of “Meningitis (inflammation of the membranes surrounding the brain and spinal cord) is a common form of meningococcal disease and is characterized by fever, severe headache, and stiff neck. Patients with meningococcal sepsis (severe illness caused by bacteria or their toxins in the blood) may present with high fever, hypotension (low blood pressure), and profound weakness. In either case, patients may develop a characteristic rash including petechiae (pinpoint red spots that do not blanch with pressure) or purpura (purple areas similar to bruises) that are caused by bleeding into the skin. Purpura fulminans (hemorrhagic condition resulting in tissue necrosis and small vessel thrombosis) can result in scarring or limb amputations. Approximately 10-14% of cases of meningococcal disease are fatal. Of patients who recover, 11-19% have permanent hearing loss, mental retardation, loss of limbs or other severe sequelae." [2] For this reason major amounts of research is being done on this bacteria and its transport mechanisms. StructureBeta BarrelThe beta barrel is a unique structure that makes this pore able to allow for transportation in and out of the gram-negative cell. This beta barrel is created with 12 anti-parallel beta-pleated sheets that have wrapped around creating anti-parellel interaction between sheet 1 and sheet 12. This creates a tube structure that transcends through the membrane of a cell creating a new environment that allows for polar molecules to move through the cell membrane and cell wall when they would have otherwise been stopped by the hydrophobic center of peptidoglycan. The start and end of the beta barrel is on the periplasmic side of the membrane and a short tight turn, , connects the alpha helix to the N-terminal beta strand. The alpha helix has its N-terminus side facing outward toward extracellular material. [3]
Alpha HelixThe alpha helix within the beta barrel is a major obstruction, which allows for regulated channel. The alpha helix corresponds to the .15nS opening that is observed and without this obstruction a 1.3nS open pore is created which allows for a much more free flowing pore. This is found to be an infrequent occurrence that could be caused by a detergent and high salt concentration. Due to this being the more infrequent type of pore it can be deduced that the internal alpha helix is what is found in vivo. The alpha helix is found internally on the N-terminus side of the protein and extends from , colored orange, leading inward toward the cytoplasm that then turns into a beta pleated sheet that creates the barrel shape. This structure is consistent with the final stage of translocation, which allows for proteins to be released into the extracellular space. The alpha helix is charged almost solely on one side. This charged side of the alpha helix is able to interact with an axial line of charged side chains that point inward from the beta barrel. Through seven salt bridges, as well as 16 hydrogen bonds and several van der Waals contacts, the alpha helix is able to interact with one side of the beta barrel.[3]
Function of Alpha HelixIn order to test the function of the alpha helix within the domain, a test was done in order to compare results of uptake within the domain in the presence of the alpha helix and without the alpha helix. An easy way to test this was an antibiotic assay. By isolating colonies strictly of gram-negative bacteria with the alpha helix, NalPβ, and isolating colonies strictly of gram-negative bacteria without the alpha helix, NalPβΔhelix, and then plating these separately. From this, it was possible to compare susceptibility to antibiotics by placing the small circular tabs of antibiotics on the plate and measuring the difference in how effective the antibiotics were in penetrating the cell. More penetration means less growth inhibition or more sensitivity to the antibiotics. When the alpha helix was removed there was much more sensitivity to antibiotics showing that the removal leads to a more open pore.[1]
Integration of the Translocator Domain into Outer MembraneOmp85 has been found in many studies to help integrate beta barrels into the outer membrane in order to allow the autotransporter to complete its duty. Due to the of the beta barrel’s hairpin loops on the extracellular side of NaIP, it is impossible for it to breach the cell membrane that is highly hydrophobic. Research on how this occurs in Neisseria meningitides is ongoing and has not been discovered yet. Yet there are many implications that a protein named Omp85 is most likely the helper protein that facilitates this. The large hydrophilic loops on the autotransporter domain might act as a recognition signal for the Omp85 complex to encompass the end of the beta barrel. From here the Omp85 complex which sits on the periplasmic side of the cell membrane is activated and creates a pore and places the beta barrel within the membrane, while preventing the hydrophilic loops from directly coming in contact with the hydrophobic cell membrane. Then the Omp85 molecule is able to integrate the beta barrel into the pore that it created, situating it permanently there. The lag time between Omp85 and the translocator exporting a protein is very small and it is hard to tell whether they can occur simultaneously or only occur simultaneously. [3]
Protein Transportation Mechanism
Interesting questions were raised on how the alpha helix in the center of the beta barrel affected the mechanism of protein transportation out of the cell. The first step to understanding what shapes of proteins can move though the pore was figured out by trying to move a disulfide bond through the pore. This was unsuccessful and led to part of the understanding that the only way that proteins can move though this pore was by being completely unfolded. Yet once inside of the extracellular material, the protein must be folded. Knowing these two crucial pieces of data, it was clear that as the protein passes through the pore it must make a transition from unfolded to folded. Due to the C-terminal end's placement on the periplasmic side of the pore it was highly unlikely that was the participating portion that effected the change in conformation of the protein as it passes through. Oppositely the N-terminal side of the pore lies on the alpha helix facing the extracellular matter, placing it in prime location to change the conformation of the passing protein. Another possible place where interaction could occur between the passing protein and the pore would be at a large hairpin loop that is on the extracellular side of the pore. This would also provide a prime placement for the initiation of protein folding.[1]
Threading ModelThe threading model could be one possible model that would allow for transportation of passenger proteins out of the cell and into extracellular material. The model has one single strand of protein entering and as it crosses to the other side it begins to fold starting with its N-terminus. The threading model is a possible explanation for how a protein would be able to fit through the narrow gap that the beta barrel and alpha helix provide. The threading model allows for one strand of the DNA to pass through the pore without being sterically hindered by the size of the alpha helix that blocks the beta barrel. Yet there are reasons why this is an unlikely model. One major reason why this seems implausible is that other research that has been done on autotransporters has shown that the last thing to leave the pore of the passenger protein is the N-terminus. So therefore how would the N-terminus start the folding if it is the last thing to leave? This also means that the autotransporter would have to secrete something in order to allow for the attraction of the N-terminus side to the pore. This was unable to be shown in artificial passenger proteins.[1]
Hairpin ModelThe Hairpin Model is a much more likely model that could accommodate for the movement across the membrane. The hairpin model allows for a hairpin loop to be created in the passenger protein and as it passes through the end of the pore a hairpin loop there interacts with the passenger protein in order to create the folding. As the protein is folded it provides energy to pull the rest of the protein through. One major problem with this model is the fact that 2 strands must fit through the protein at once and with the alpha helix that is an impossible fit. The model describes a fix to this problem with the destruction of the alpha helix and recreation. When the alpha helix is nonexistent the hydrogens that typically face inward and interact with the alpha helix face toward one another or even outward which expands the barrel as well as making it much more flexible. This model suggests that folding and translocation are interdependent and happen simultaneously. The problem with this method is there is no mechanism for how the alpha helix is created or dismantled and where it goes.[1]
Alternative TheoryOne alternative theory argues that the beta barrel is actually not used as a protein secretion pore at all. As Omp85 encompasses the end of the beta barrel it travels toward the cell membrane as if to place the autotransporter into the cell membrane yet instead of placing it, Omp85 continues through into the extracellular material. As the translocator is being carried toward the cell membrane it is able to pick up a passenger protein using its loose C-terminus end that would have faced inward toward the perIplasm. Then all three the, the Omp85, the translocator, and the passenger protein, are transported to extracellular material through the pore that Omp85 is able to create. Then they all dissociate away from one another, which frees the passenger protein. This is another possibility for how the translocator is able to transport passenger protein out of the cell yet changes the view of the translocator all-together. If this is in fact the way that passenger proteins leave the cell then NaIP is not an autotransporter at all. An autotransporter, just as it sounds, autotransports, meaning that the protein pulls itself through as it is folded on the opposite side of the cell. As plausible as this seems, it would mean a major change in the way that this translocator protein is classified. [1]
Similar Structure in Other ProteinsRecent research has showed that there are possible conserved features to this pore and pores in other types of gram-negative bacteria. Autotransporters that also have the conserved structure of an alpha helix directly preceding the beta core include: AidaI of E. coli, BrkA of B. pertussis, Hap of Hemophilus influenzae and IgA protease and App of N. meningitidis. Much of the these proteins show low conservation within their alpha helixes yet they all have a long traversing alpha helix that leads into the 12 sheeted beta barrel. Due to the amount of research being done on the Neisseria meningitidis' NalPβ protein, its crystal structure is being used in order to model autotransporter secretion. [1] |
| ||||||||||
3D structure of NalP3D structure of NalP
ReferencesReferences
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Oomen, Clasien J., Patrick Van Gelder, Peter Van Ulsen, Maya Feijen, Jan Tommassen, and Piet Gros. "Structure of the Translocator Domain of a Bacterial Autotransporter." Www.ncbi.nlm.nih.gov. The EMBO Journal, 11 Mar. 2004. Web. 6 Nov. 2012. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC381419/>.
- ↑ "Neisseria Meningitidis." Neisseria Meningitidis. Georgia Department of Public Health, n.d. Web. 13 Nov. 2012. <http://health.state.ga.us/epi/bacterial/path-neisseria.asp>.
- ↑ 3.0 3.1 3.2 Klauser T, Kramer J, Otzelberger K, Pohlner J, Meyer TF. Characterization of the Neisseria Iga beta-core. The essential unit for outer membrane targeting and extracellular protein secretion. J Mol Biol. 1993 Dec 5;234(3):579-93. PMID:8254661 doi:http://dx.doi.org/S0022-2836(83)71613-X