C-di-GMP specific phosphodiesterases
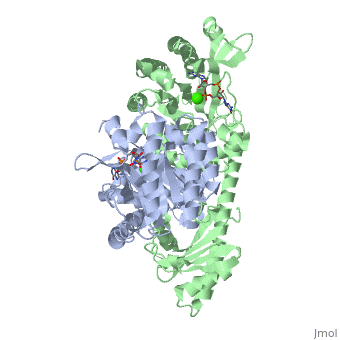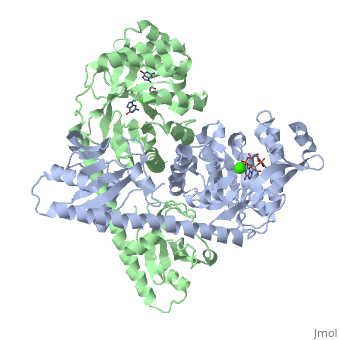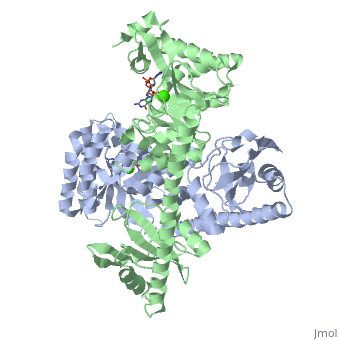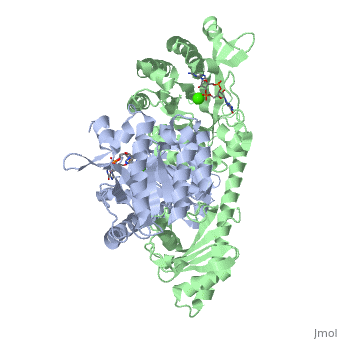
YkuI is a dimer with each protomer composed of an (with TIM barrel fold), a long , and a . The is situated at the C-terminal end of the central β-barrel of the EAL domain. The residues of the active site are well conserved, including E33 and L35 of the EAL signature motif. The is bound flat upon the actve site with the divalent metal (here Ca++) being sandwiched between binding site and C-di-GMP. BlrP1(3gg0)
Additional Resources
|
| ||||||||||
3D structure of C-di-GMP phosphodiesterase3D structure of C-di-GMP phosphodiesterase
ReferencesReferences
- ↑ Minasov G, Padavattan S, Shuvalova L, Brunzelle JS, Miller DJ, Basle A, Massa C, Collart FR, Schirmer T, Anderson WF. Crystal structures of YkuI and its complex with second messenger cyclic Di-GMP suggest catalytic mechanism of phosphodiester bond cleavage by EAL domains. J Biol Chem. 2009 May 8;284(19):13174-84. Epub 2009 Feb 24. PMID:19244251 doi:10.1074/jbc.M808221200
BlrP1 structure 3gg0: [xtra 1]
- ↑ Barends TR, Hartmann E, Griese JJ, Beitlich T, Kirienko NV, Ryjenkov DA, Reinstein J, Shoeman RL, Gomelsky M, Schlichting I. Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature. 2009 Jun 18;459(7249):1015-8. PMID:19536266 doi:10.1038/nature07966