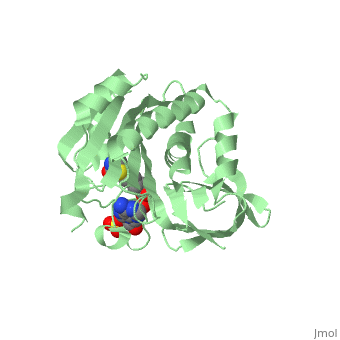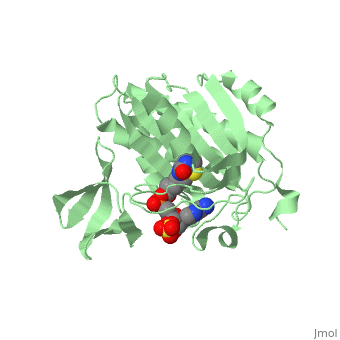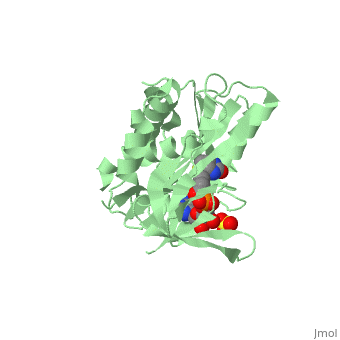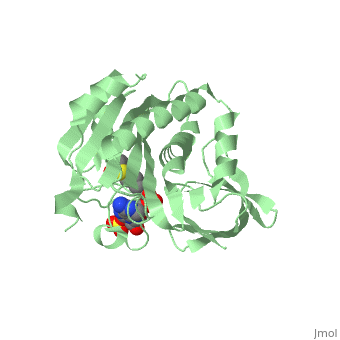4op0
Crystal structure of biotin protein ligase (RV3279C) of Mycobacterium tuberculosis, complexed with biotinyl-5'-AMPCrystal structure of biotin protein ligase (RV3279C) of Mycobacterium tuberculosis, complexed with biotinyl-5'-AMP
Structural highlights
FunctionBIRA_MYCTU Catalyzes the transfer of biotin onto a conserved lysine residue of the biotin carboxyl carrier protein (BCCP) domain of acetyl-CoA carboxylase and converts it to active holo-BCCP (PubMed:18509457, PubMed:24723382). Forms an acyl-adenylate intermediate (PubMed:18509457, PubMed:24723382). Cannot use GTP or desthiobiotin (PubMed:18509457).[1] [2] Publication Abstract from PubMedProtein biotinylation, a rare form of post-translational modification, is found in enzymes required for lipid biosynthesis. In mycobacteria, this process is essential for the formation of their complex and distinct cell wall and has become a focal point of drug discovery approaches. The enzyme responsible for this process, biotin protein ligase, substantially varies in different species in terms of overall structural organization, regulation of function and substrate specificity. To advance the understanding of the molecular mechanism of biotinylation in Mycobacterium tuberculosis we have biochemically and structurally characterized the corresponding enzyme. We report the high-resolution crystal structures of the apo-form and reaction intermediate biotinyl-5'-AMP-bound form of M. tuberculosis biotin protein ligase. Binding of the reaction intermediate leads to clear disorder-to-order transitions. We show that a conserved lysine, Lys138, in the active site is essential for biotinylation. Active site conformational changes upon reaction intermediate biotinyl-5'-AMP binding in biotin protein ligase from Mycobacterium tuberculosis.,Ma Q, Akhter Y, Wilmanns M, Ehebauer MT Protein Sci. 2014 Apr 9. doi: 10.1002/pro.2475. PMID:24723382[3] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||