3ve1
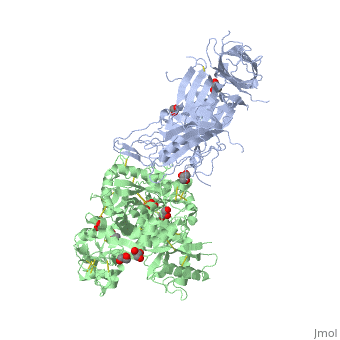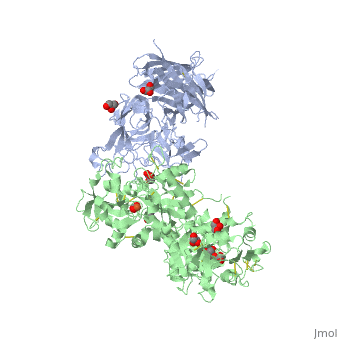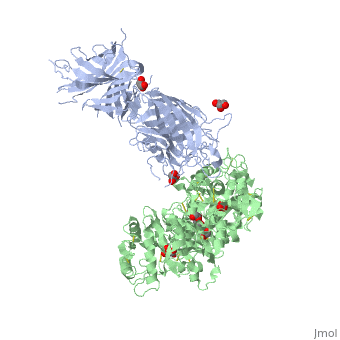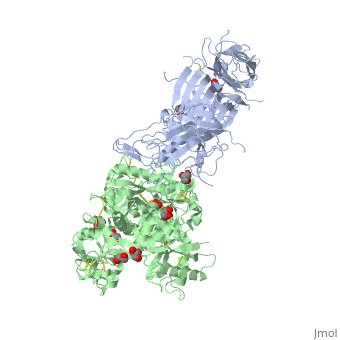
The 2.9 angstrom crystal structure of Transferrin binding protein B (TbpB) from serogroup B M982 Neisseria meningitidis in complex with human transferrinThe 2.9 angstrom crystal structure of Transferrin binding protein B (TbpB) from serogroup B M982 Neisseria meningitidis in complex with human transferrin
Structural highlights
DiseaseTRFE_HUMAN Defects in TF are the cause of atransferrinemia (ATRAF) [MIM:209300. Atransferrinemia is rare autosomal recessive disorder characterized by iron overload and hypochromic anemia.[1] [2] FunctionTRFE_HUMAN Transferrins are iron binding transport proteins which can bind two Fe(3+) ions in association with the binding of an anion, usually bicarbonate. It is responsible for the transport of iron from sites of absorption and heme degradation to those of storage and utilization. Serum transferrin may also have a further role in stimulating cell proliferation. Publication Abstract from PubMedNeisseria meningitidis, the causative agent of bacterial meningitis, acquires the essential element iron from the host glycoprotein transferrin during infection through a surface transferrin receptor system composed of proteins TbpA and TbpB. Here we present the crystal structures of TbpB from N. meningitidis in its apo form and in complex with human transferrin. The structure reveals how TbpB sequesters and initiates iron release from human transferrin. The structural basis of transferrin sequestration by transferrin-binding protein B.,Calmettes C, Alcantara J, Yu RH, Schryvers AB, Moraes TF Nat Struct Mol Biol. 2012 Feb 19;19(3):358-60. doi: 10.1038/nsmb.2251. PMID:22343719[3] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||