3mhs
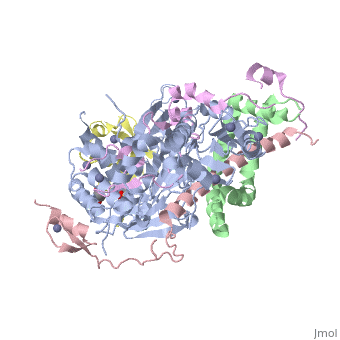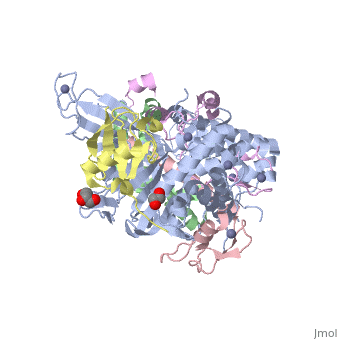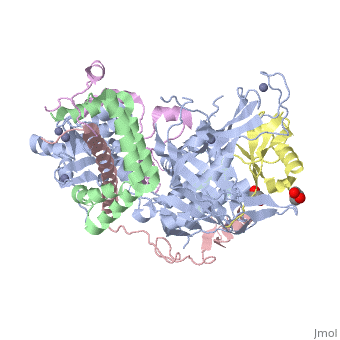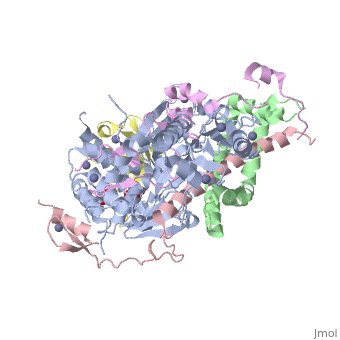
Structure of the SAGA Ubp8/Sgf11/Sus1/Sgf73 DUB module bound to ubiquitin aldehydeStructure of the SAGA Ubp8/Sgf11/Sus1/Sgf73 DUB module bound to ubiquitin aldehyde
Structural highlights
Function[UBP8_YEAST] Functions as histone deubiquitinating component of the transcription regulatory histone acetylation (HAT) complexes SAGA and SLIK. SAGA is involved in RNA polymerase II-dependent transcriptional regulation of approximately 10% of yeast genes. At the promoters, SAGA is required for recruitment of the basal transcription machinery. It influences RNA polymerase II transcriptional activity through different activities such as TBP interaction (SPT3, SPT8 and SPT20) and promoter selectivity, interaction with transcription activators (GCN5, ADA2, ADA3 and TRA1), and chromatin modification through histone acetylation (GCN5) and deubiquitination (UBP8). SAGA acetylates nucleosomal histone H3 to some extent (to form H3K9ac, H3K14ac, H3K18ac and H3K23ac). SAGA interacts with DNA via upstream activating sequences (UASs). SLIK is proposed to have partly overlapping functions with SAGA. It preferentially acetylates methylated histone H3, at least after activation at the GAL1-10 locus. Together with SGF11, is required for histone H2B deubiquitination.[1] [2] [3] [SGF11_YEAST] Component of the transcription regulatory histone acetylation (HAT) complex SAGA. SAGA is involved in RNA polymerase II-dependent transcriptional regulation of approximately 10% of yeast genes. At the promoters, SAGA is required for recruitment of the basal transcription machinery. It influences RNA polymerase II transcriptional activity through different activities such as TBP interaction (SPT3, SPT8 and SPT20) and promoter selectivity, interaction with transcription activators (GCN5, ADA2, ADA3 and TRA1), and chromatin modification through histone acetylation (GCN5) and deubiquitination (UBP8). SAGA acetylates nucleosomal histone H3 to some extent (to form H3K9ac, H3K14ac, H3K18ac and H3K23ac). SAGA interacts with DNA via upstream activating sequences (UASs). SGF11 is involved in transcriptional regulation of a subset of SAGA-regulated genes. Within the SAGA complex, participates in a subcomplex with SUS1, SGF73 and UBP8 required for deubiquitination of H2B and for the maintenance of steady-state H3 methylation levels. It is required to recruit UBP8 and SUS1 into the SAGA complex.[4] [5] [SUS1_YEAST] Involved in mRNA export coupled transcription activation by association with both the TREX-2 and the SAGA complexes. The transcription regulatory histone acetylation (HAT) complex SAGA is involved in RNA polymerase II-dependent regulation of approximately 10% of yeast genes. At the promoters, SAGA is required for recruitment of the basal transcription machinery. It influences RNA polymerase II transcriptional activity through different activities such as TBP interaction (SPT3, SPT8 and SPT20) and promoter selectivity, interaction with transcription activators (GCN5, ADA2, ADA3 and TRA1), and chromatin modification through histone acetylation (GCN5) and deubiquitination (UBP8). SUS1 forms a distinct functional SAGA module with UBP8, SGF11 and SGF73 required for deubiquitination of H2B and for the maintenance of steady-state H3 methylation levels. The TREX-2 complex functions in docking export-competent ribonucleoprotein particles (mRNPs) to the nuclear entrance of the nuclear pore complex (nuclear basket), by association with components of the nuclear mRNA export machinery (MEX67-MTR2 and SUB2) in the nucleoplasm and the nucleoporin NUP1 at the nuclear basket. TREX-2 participates in mRNA export and accurate chromatin positioning in the nucleus by tethering genes to the nuclear periphery. SUS1 has also a role in mRNP biogenesis and maintenance of genome integrity through preventing RNA-mediated genome instability. Finally SUS1 has a role in response to DNA damage induced by methyl methane sulfonate (MMS) and replication arrest induced by hydroxyurea.[6] [7] [8] [9] [10] [11] [12] [SGF73_YEAST] Functions as component of the transcription regulatory histone acetylation (HAT) complex SAGA. SAGA is involved in RNA polymerase II-dependent transcriptional regulation of approximately 10% of yeast genes. At the promoters, SAGA is required for recruitment of the basal transcription machinery. It influences RNA polymerase II transcriptional activity through different activities such as TBP interaction (SPT3, SPT8 and SPT20) and promoter selectivity, interaction with transcription activators (GCN5, ADA2, ADA3 and TRA1), and chromatin modification through histone acetylation (GCN5) and deubiquitination (UBP8). SAGA acetylates nucleosomal histone H3 to some extent (to form H3K9ac, H3K14ac, H3K18ac and H3K23ac). SAGA interacts with DNA via upstream activating sequences (UASs). Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedSAGA is a transcriptional coactivator complex that is conserved across eukaryotes and performs multiple functions during transcriptional activation and elongation. One role is deubiquitination of histone H2B, and this activity resides in a distinct subcomplex called the deubiquitinating module (DUBm), which contains the ubiquitin-specific protease Ubp8, bound to Sgf11, Sus1, and Sgf73. The deubiquitinating activity depends on the presence of all four DUBm proteins. We report here the 1.90 angstrom resolution crystal structure of the DUBm bound to ubiquitin aldehyde, as well as the 2.45 angstrom resolution structure of the uncomplexed DUBm. The structure reveals an arrangement of protein domains that gives rise to a highly interconnected complex, which is stabilized by eight structural zinc atoms that are critical for enzymatic activity. The structure suggests a model for how interactions with the other DUBm proteins activate Ubp8 and allows us to speculate about how the DUBm binds to monoubiquitinated histone H2B in nucleosomes. Structural insights into the assembly and function of the SAGA deubiquitinating module.,Samara NL, Datta AB, Berndsen CE, Zhang X, Yao T, Cohen RE, Wolberger C Science. 2010 May 21;328(5981):1025-9. Epub 2010 Apr 15. PMID:20395473[13] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||||||||