2-isopropylmalate synthase
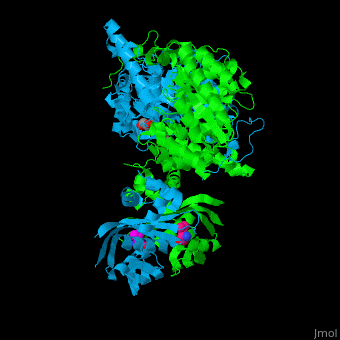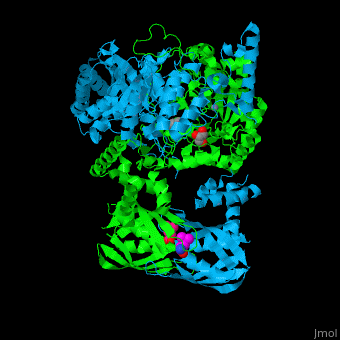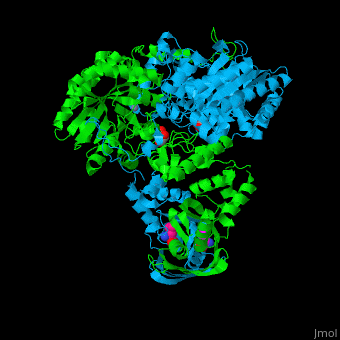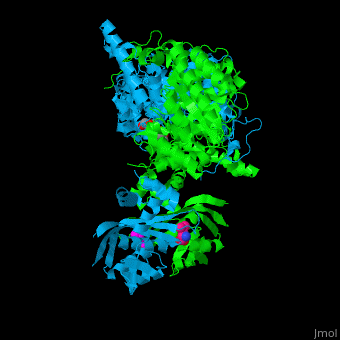
Function2-isopropylmalate synthase (LeuA) or α-isopropylmalate synthase catalyzes the reversible conversion of 3-methyl-2-oxobutanoate and acetyl-CoA to 2-isopropylmalate. LeuA participates in the biosynthesis of leucine and pyruvate metabolism[1]. It is involved in the second step of the leucine biosynthetic pathway, catalyzing the formation of 2-isopropylmalate from acetyl-CoA and α-ketoisovalerate. The leucine biosynthetic pathway is found in bacteria, fungi, plants, and some archaea. It allows these organisms to synthesize leucine, an essential amino acid that cannot be produced by animals and must be obtained through the diet. The reaction catalyzed by 2-isopropylmalate synthase involves condensing acetyl-CoA with α-ketoisovalerate, resulting in the formation of 2-isopropylmalate. This reaction is a key step in the conversion of α-ketoisovalerate into α-isopropylmalate, which is subsequently converted to leucine through a series of enzymatic reactions. 2-Isopropylmalate synthase is a pyrocatechol-4,5-dioxygenase-like enzyme and requires divalent metal ions, such as manganese or magnesium, as cofactors for its activity. The enzyme is typically composed of multiple subunits and can vary in structure and organization among different organisms. Understanding the structure and function of 2-isopropylmalate synthase is important for studying the biosynthesis of leucine and related amino acids. It is also a potential target for the development of antimicrobial agents, as the leucine biosynthetic pathway is unique to microorganisms and absent in animals, making it an attractive target for selective inhibition. Structural highlights. LeuA structure is composed of 2 major domains - the catalytic N-terminal which shows a TIM barrel conformation and the regulatory C-terminal. The 2 domains are separated by subdomains I and II and a short flexible hinge region between the 2 subdomains. The catalytic domain binds the substrate and the [2]. Water molecules are shown as red spheres. . 3D structures of 2-isopropylmalate synthase2-isopropylmalate synthase 3D structures
|
| ||||||||||
ReferencesReferences
- ↑ de Carvalho LP, Blanchard JS. Kinetic and chemical mechanism of alpha-isopropylmalate synthase from Mycobacterium tuberculosis. Biochemistry. 2006 Jul 25;45(29):8988-99. PMID:16846242 doi:http://dx.doi.org/10.1021/bi0606602
- ↑ Koon N, Squire CJ, Baker EN. Crystal structure of LeuA from Mycobacterium tuberculosis, a key enzyme in leucine biosynthesis. Proc Natl Acad Sci U S A. 2004 Jun 1;101(22):8295-300. Epub 2004 May 24. PMID:15159544 doi:10.1073/pnas.0400820101