1w06
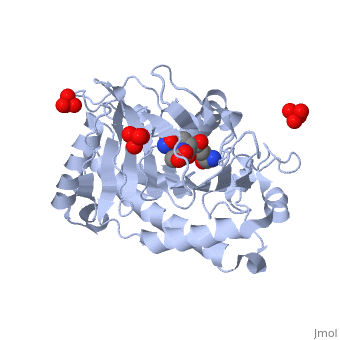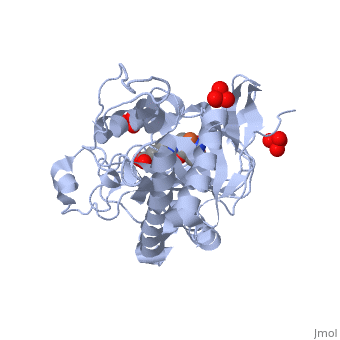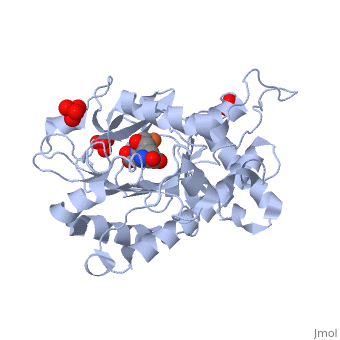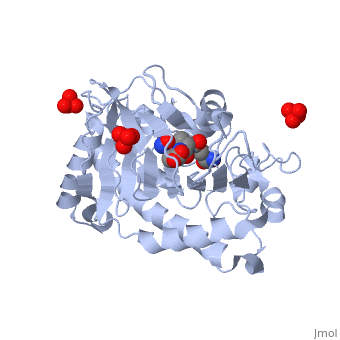
Isopenicillin N Synthase Aminoadipoyl-Cysteinyl-Alanine-Fe NO ComplexIsopenicillin N Synthase Aminoadipoyl-Cysteinyl-Alanine-Fe NO Complex
Structural highlights
FunctionIPNA_EMENI Isopenicillin N synthase; part of the gene cluster that mediates the biosynthesis of penicillin, the world's most important antibiotic (PubMed:3319778, PubMed:11755401). IpnA catalyzes the cyclization of the tripeptide N-[(5S)-5-amino-5-carboxypentanoyl]-L-cysteinyl-D-valine (LLD-ACV or ACV) to form isopenicillin N (IPN) that contains the beta-lactam nucleus (PubMed:3319778, PubMed:11755401, PubMed:28703303). The penicillin biosynthesis occurs via 3 enzymatic steps, the first corresponding to the production of the tripeptide N-[(5S)-5-amino-5-carboxypentanoyl]-L-cysteinyl-D-valine (LLD-ACV or ACV) by the NRPS acvA. The tripeptide ACV is then cyclized to isopenicillin N (IPN) by the isopenicillin N synthase ipnA that forms the beta-lactam nucleus. Finally, the alpha-aminoadipyl side chain is exchanged for phenylacetic acid by the isopenicillin N acyltransferase penDE to yield penicillin in the peroxisomal matrix (By similarity).[UniProtKB:P08703][1] [2] [3] Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedIsopenicillin N synthase (IPNS), a non-heme iron(II)-dependent oxidase, catalyzes conversion of the tripeptide delta-(l-alpha-aminoadipoyl)-l-cysteinyl-d-valine (ACV) to bicyclic isopenicillin N (IPN), concomitant with the reduction of dioxygen to two molecules of water. Incubation of the "truncated"substrate analogues delta-(l-alpha-aminoadipoyl)-l-cysteinyl-glycine (ACG) and delta-(l-alpha-aminoadipoyl)-l-cysteinyl-d-alanine (ACA) with IPNS has previously been shown to afford acyclic products, in which the substrate cysteinyl residue has undergone a two-electron oxidation. We report X-ray crystal structures for the anaerobic IPNS/Fe(II)/ACG and IPNS/Fe(II)/ACA complexes, both in the absence and presence of the dioxygen analogue nitric oxide. The overall protein structures are very similar to those of the corresponding IPNS/Fe(II)/ACV complexes; however, significant differences are apparent in the vicinity of the active site iron. The structure of the IPNS/Fe(II)/ACG complex reveals that the C-terminal carboxylate of this substrate is oriented toward the active site iron atom, apparently hydrogen-bonded to an additional water ligand at the metal; this is a different binding mode to that observed in the IPNS/Fe(II)/ACV complex. ACA binds to the metal in a manner that is intermediate between those observed for ACV and ACG. The addition of NO to these complexes initiates conformational changes such that both the IPNS/Fe(II)/ACG/NO and IPNS/Fe(II)/ACA/NO structures closely resemble the IPNS/Fe(II)/ACV/NO complex. These results further demonstrate the feasibility of metal-centered rearrangements in catalysis by non-heme iron enzymes and provide insight into the delicate balance between hydrophilic-hydrophobic interactions and steric effects in the IPNS active site. Structural studies on the reaction of isopenicillin N synthase with the truncated substrate analogues delta-(L-alpha-aminoadipoyl)-L-cysteinyl-glycine and delta-(L-alpha-aminoadipoyl)-L-cysteinyl-D-alanine.,Long AJ, Clifton IJ, Roach PL, Baldwin JE, Rutledge PJ, Schofield CJ Biochemistry. 2005 May 3;44(17):6619-28. PMID:15850395[4] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||