1fep
FERRIC ENTEROBACTIN RECEPTORFERRIC ENTEROBACTIN RECEPTOR
Structural highlights
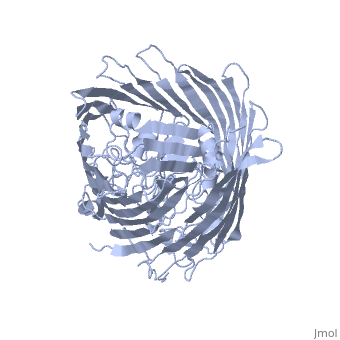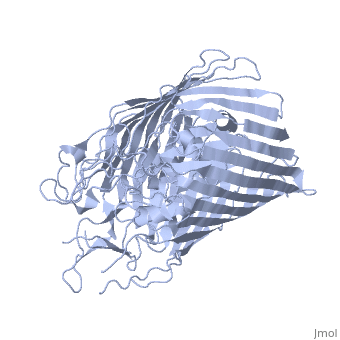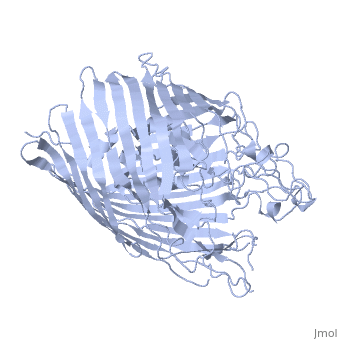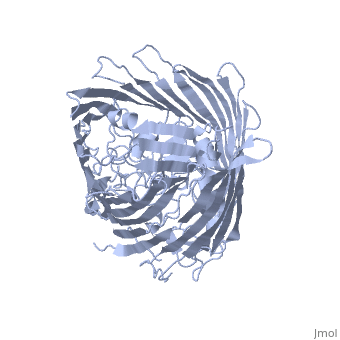
FunctionFEPA_ECOLI This protein is involved in the initial step of iron uptake by binding ferrienterobactin (Fe-ENT), an iron chelatin siderophore that allows E.coli to extract iron from the environment. FepA also acts as a receptor for colicins B and D. Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedIntegral outer membrane receptors for iron chelates and vitamin B12 carry out specific ligand transport against a concentration gradient. Energy for active transport is obtained from the proton-motive force of the inner membrane through physical interaction with TonB-ExbB-ExbD, an inner membrane complex. Here we report the crystal structure of an active transport, outer membrane receptor at 2.4 A resolution. Two distinct functional domains are revealed: (i) a 22-stranded beta-barrel that spans the outer membrane and contains large extracellular loops which appear to function in ligand binding; and (ii) a globular N-terminal domain that folds into the barrel pore, inhibiting access to the periplasm and contributing two additional loops for potential ligand binding. These loops could provide a signaling pathway between the processes of ligand recognition and TonB-mediated transport. The blockage of the pore suggests that the N-terminal domain must undergo a conformational rearrangement to allow ligand transport into the periplasm. Crystal structure of the outer membrane active transporter FepA from Escherichia coli.,Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J Nat Struct Biol. 1999 Jan;6(1):56-63. PMID:9886293[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||