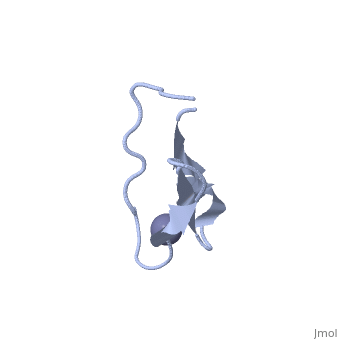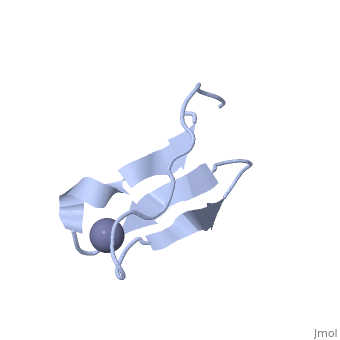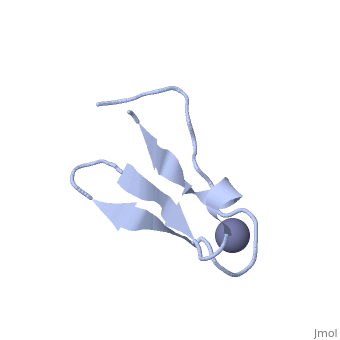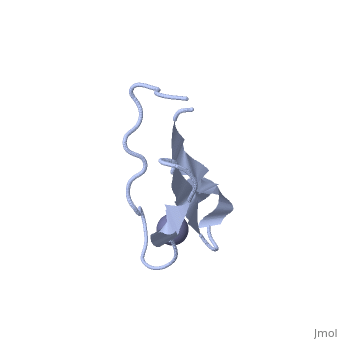1dfe
NMR STRUCTURE OF RIBOSOMAL PROTEIN L36 FROM THERMUS THERMOPHILUSNMR STRUCTURE OF RIBOSOMAL PROTEIN L36 FROM THERMUS THERMOPHILUS
Structural highlights
FunctionEvolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedWe have determined the solution NMR structure of the ribosomal protein L36 from Thermus thermophilus. L36 is the smallest protein in the large subunit of the prokaryotic ribosome. The sequence contains three completely conserved cysteine residues and one conserved histidine residue in a C-X(2)-C-X(12)-C-X(4)-H motif. Extended X-ray absorption fine structure spectroscopy was used to confirm that a purified L36 sample contains an equimolar amount of zinc. The structure of L36 was determined using simulated annealing based on NOE distance restraints, dihedral angle restraints and hydrogen bond distance restraints derived from NMR spectra of (15)N-labeled and non-labeled L36 samples at pH 7 and 12 degrees C, and by imposing tetrahedral zinc ion coordination geometry. The L36 fold is characterized by a triple-stranded antiparallel beta-sheet with the zinc-binding site at one end. The structure of the zinc site is well-determined and shows that the three cysteine sulphur atoms are supported by hydrogen bonds to backbone amide protons. The conserved histidine residue is located in a short 3(10)-helix and coordinates zinc by the N(delta1) atom. The electrostatic surface potential and location of conserved Arg, Lys and His side-chains suggest a large continuous L36-rRNA interaction interface. The folding topology as well as position and conformation of many conserved side-chains in L36 are very similar to those of zinc-ribbon domains found in the archaeal transcription factor TFIIB N terminus and the eukaryal transcription elongation factor hTFIIS C terminus. Given the relative antiquity of the ribosome it is possible that L36 reflects the parent of transcription-related zinc ribbons. The solution structure of ribosomal protein L36 from Thermus thermophilus reveals a zinc-ribbon-like fold.,Hard T, Rak A, Allard P, Kloo L, Garber M J Mol Biol. 2000 Feb 11;296(1):169-80. PMID:10656825[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||